Abstract
Strigolactones (SLs) constitute a class of plant hormones that regulate many aspects of plant development, including repressing tillering in rice (Oryza sativa). However, how SL pathways are regulated is still poorly understood. Here, we describe a rice mutant dwarf and high tillering1 (dht1), which exhibits pleiotropic phenotypes (such as dwarfism and increased tiller numbers) similar to those of mutants defective in SL signaling. We show that DHT1 encodes a monocotyledon-specific hnRNP-like protein that acts as a previously unrecognized intron splicing factor for many precursor mRNAs (pre-mRNAs), including for the SL receptor gene D14. We find that the dht1 (DHT1I232F) mutant protein is impaired in its stability and RNA binding activity, causing defective splicing of D14 pre-mRNA and reduced D14 expression, and consequently leading to the SL signaling-defective phenotypes. Overall, our findings deepen our understanding of the functional diversification of hnRNP-like proteins and establish a connection between posttranscriptional splicing and SL signaling in the regulation of plant development.
In a Nutshell.
Background: Rice is one of the staple crops in the world, feeding more than half of the world’s population. Plant height, tiller number, and panicle morphology, which are controlled by complex genetic networks, are important factors that affect plant architecture and determine rice grain yield. Strigolactones (SLs) constitute a class of plant hormones that regulate many aspects of plant development, including repressing tillering in rice. However, how the SL pathway is regulated is still poorly understood.
Question: We wanted to know if there are any unknown mechanisms involved in the regulation of SL signaling. By a forward genetic approach, we identified a rice dwarf and high tillering1 (dht1) mutant defective in SL signaling and wanted to know the detailed role of DHT1 gene in regulating SL signaling.
Findings: We found that dht1 exhibits similar pleiotropic phenotypes to mutant defective in SL signaling. We showed that DHT1 encodes a monocotyledon-specific hnRNP-like protein localized in the nuclear speckle. We found that DHT1, as a component of the core splicing factor U1 snRNP, cooperates with various SR proteins to regulate the intron splicing of precursor mRNA (pre-mRNA) of many genes. Interestingly, we found that DHT1 regulates the pre-mRNA splicing of the SL receptor gene D14. Furthermore, the DHT1I232F mutant protein is impaired in its stability and RNA binding activity, causing defective splicing of D14 pre-mRNA and reduced D14 expression, and consequently leading to the SL signaling-defective phenotypes. Overall, our findings reveal a connection between posttranscriptional splicing and SL signaling in regulating plant development.
Next steps: DHT1 regulates rice architecture as an important splicing factor. Moreover, DHT1 has many target genes involved in multiple development processes of plants. The coupling mechanism between the splicing machinery and these plant development processes should be studied in future.
Introduction
Rice (Oryza sativa) is one of the most important food crops in the world, and more than half of the world’s population eats rice as the staple food. Plant height, tiller number, and panicle morphology are important factors that determine rice grain yield, and they are regulated by complex genetic networks (Wang and Li, 2008; Wang et al., 2018). Recently, strigolactones (SLs) were identified as a novel class of terpenoid lactone phytohormone that regulates diverse aspects of plant growth and development, including repressing the outgrowth of axillary buds, promoting internode elongation, stem thickening, root growth, flower development, and leaf senescence (Umehara et al., 2008; Waters et al., 2017). SLs are synthesized from carotenoids in plants through a series of metabolic processes (Al-Babili and Bouwmeester, 2015; Hou et al., 2016). Recent studies showed that in rice, D27 encodes a β-carotene isomerase in charge of converting all-trans-β-carotene into 9-cis-β-carotene, which is subsequently catalyzed by carotenoid cleavage dioxygenase (encoded by CCD7/D17 and CCD8/D10) to yield the SL precursor carlactone (Zou et al., 2006; Arite et al., 2007; Lin et al., 2009; Alder et al., 2012). SLs are perceived by an ɑ/β-hydrolase fold family protein encoded by DWARF14 (D14), and activation of SL signaling leads to targeted degradation of a central repressor D53 by the D14-SCFD3 E3 ubiquitin ligase complex through the 26S proteasome-mediated ubiquitin pathway (Ishikawa et al., 2005; Arite et al., 2009; Jiang et al., 2013; Zhou et al., 2013), thus unleashing SL responses. In addition, recent studies showed that D53 represses the transcription activation activity of OsSPL14 (IPA1) to promote tiller bud outgrowth and D53 can balance the expression of D53 through negative feedback regulation (Song et al., 2017); OsTB1, as the downstream of SLs, interacts with OsMADS57 to reduce OsMADS57’s inhibition of D14 transcription to control tillering (Guo et al., 2013). Moreover, it was also shown that D14 expression is activated by RNA-directed DNA methylation (Xu et al., 2020). Despite the progress, additional mechanisms regulating SLs signaling and plant architecture likely exist and await elucidation.
Precursor mRNA (pre-mRNA) splicing is a fundamental process that regulates gene expression. Pre-mRNA splicing is catalyzed by a large spliceosome complex, which consists of five core components of small nuclear ribonucleoproteins (U1, U2, U5, and U4/U6 snRNPs) and numerous non-snRNPs, such as members of serine–arginine-rich (SR) proteins and heterogeneous nuclear ribonucleoprotein (hnRNP) family (Wahl et al., 2009; Will and Luhrmann, 2011; Hoskins and Moore, 2012). Each snRNP has its own unique small nuclear RNA (snRNA) and can be step-wisely assembled into complex particles on the primal RNA substrate with corresponding subunit proteins in nuclear speckles (Rino and Carmo-Fonseca, 2009; Mao et al., 2011). During intron splicing, a large number of assistant proteins including SR proteins and hnRNP family are required to ensure accurate recognition of the splicing site by the precatalytic spliceosome, and subsequently guarantee the fidelity of the splicing by activated spliceosome (Blencowe, 2006; He and Smith, 2009; Wahl et al., 2009). The hnRNP-like proteins contain one or more conserved RNA recognition motifs (RRMs) or K homology domains, as well as auxiliary domains (Lorkovic et al., 2000; Lorkovic and Barta, 2002; Maris et al., 2005). Plant hnRNP-like proteins are involved in various aspects of posttranscriptional regulation of mRNAs and perform plant-specific biological functions, such as response to environmental stress, flowering, and circadian rhythms (Schmal et al., 2013; Yeap et al., 2014; Zhang et al., 2015; Wu et al., 2016; Tian et al., 2018; Chang et al., 2021). However, how pre-mRNA splicing and hnRNPs regulate SL signaling remains largely unclear, particularly in crop plants.
Here, we identified a dwarf and high tillering1 (dht1) rice mutant with pleiotropic phenotypes including dwarfism, more tillers, smaller organ (leaf, panicle, and grain) and shorter roots. Further, we found that DHT1 encodes a hnRNP-like protein, which can bind to poly(U) and some snRNA, and interact with SR proteins and U1-70K (a U1 snRNP subunit), indicating that DHT1 is involved in pre-mRNA splicing as a U1 snRNP component. Phylogenetic analysis shows that DHT1 belongs to a previously unrecognized monocotyledon-specific clade of UBA2-like protein family and no member in this clade has been functionally characterized yet. Further, we found that DHT1 can bind to the transcripts of pre-mRNAs of many plant genes, including D14. We demonstrate that DHT1 promotes the splicing of D14 pre-mRNA to provide enough D14 protein to induce the degradation of D53 protein and proper SL signaling.
Results
The phenotypes of dht1 mutant and map-based cloning of DHT1
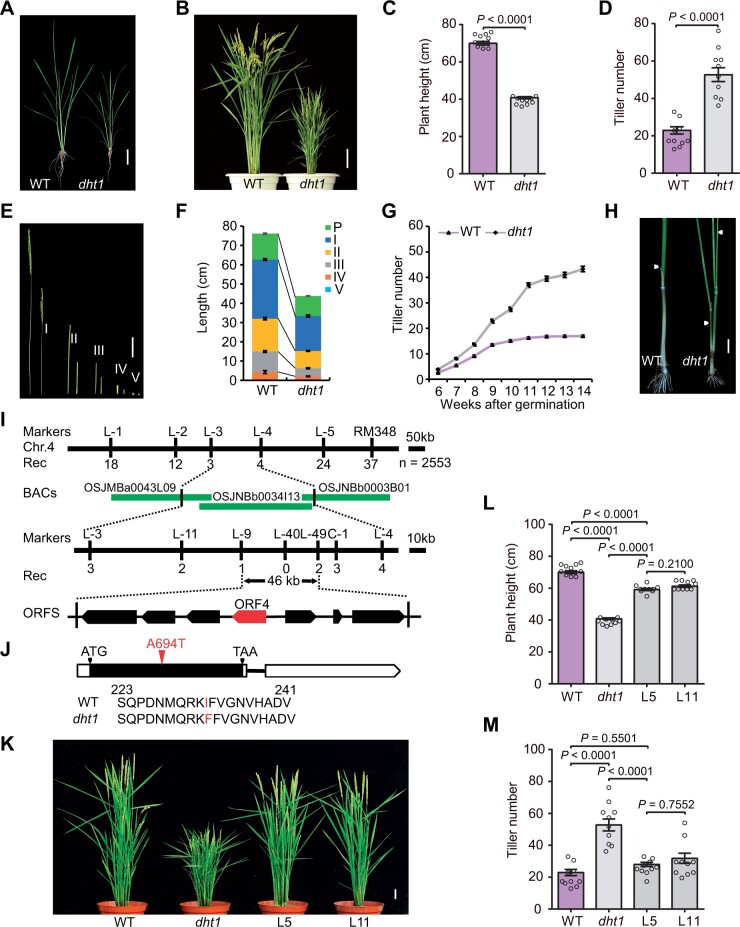
In a search for factors regulating plant architecture in rice, we identified the dht1 mutant from a tissue culture population of the japonica rice cv Kitaake. At the seedling stage, the dht1 mutant displayed retarded growth and a smaller root system compared with the wild-type (WT) plant, although the tiller number of dht1 was not notably different from WT at this stage (Figure 1A). However, at the heading stage, the dht1 mutant had reduced height and more tillers compared with WT (Figure 1, B–D). The reduced height of the mutant was mainly caused by shortened internodes (Figure 1, E and F). Histological analysis showed that the dht1 mutant had smaller stem cells with reduced cell width and length compared with WT (Supplemental Figure S1, A–E), but the cell numbers were largely comparable between WT and the dht1 mutant (Supplemental Figure S1F). In addition, the dht1 mutant plants also had smaller panicles, pistils, stamens, and grains compared with WT (Supplemental Figure S2). At the 14th week after germination, the total tiller number of dht1 was about three times that of WT, resulting from increased tillers in both lower and upper nodes (Figure 1, G and H).
Figure 1.
Phenotypes of dht1 mutant and map-based cloning of DHT1. A and B, Phenotypes of WT and dht1 plants at the sixth leaf stage (A) or the heading stage (B). Scale bars, 5 cm (A), 10 cm (B). C and D, Comparison of plant height and tiller number between WT and dht1 plants. Data are means ± sem (n = 10) and P-values are from two-sided Student’s t test. E and F, Comparison of internode length between WT (left) and dht1 plants (right). P, I, II, III, IV, and V indicate the panicle, the first, the second, the third, the fourth, and the fifth internode, respectively. Scale bars, 5 cm (E). Data in (F) are means ± sem (n = 10). G, Comparison of tiller number between WT and dht1 plants from 6th week to 14th week. Data are means ± sem (n = 50). H, Comparison of tillers on the elongated upper nodes between WT and dht1 plants. White arrowheads indicate the un-elongated tiller buds (WT) and the elongated tillers (dht1) on the elongated upper nodes. Scale bars, 2 cm. I, The dht1 locus was mapped on chromosome 4. Molecular markers and recombinant numbers are shown above and below the line, respectively. The red arrow denotes the candidate ORF. J, Mutation in the DHT1 gene. In the DHT1 gene structure diagram, the white and black boxes represent untranslated regions and CDS, respectively; the black line between boxes represents intron. ATG and TAA represent the start and stop codon, respectively. The A-to-T nucleotide substitution at 694 bp from the ATG initiation code and corresponding amino acid substitution of protein in the dht1 mutant are highlighted in red fonts. K, Complementation test by introducing the full-length DHT1 genomic fragment into the dht1 mutant. L5 and L11 are two independent transgenic lines. Scale bars, 5 cm. L and M, Comparison of plant height and tiller number between WT, dht1 and two complemented transgenic lines. Data are means ± sem (n = 10) and P-values are from one-way ANOVA test with Tukey’s correction.
To clone the causal gene, we crossed the dht1 mutant with its WT, Kitaake, and generated an F2 population. Progeny segregation in the F2 population showed that the ratio of the normal plants to mutant plants was 171:44, close to 3:1 (χ2 = 2.358, P > 0.05), indicating that dht1 is a single recessive mutation. We constructed an F2 mapping population by crossing dht1 with the japonica variety IRAT129 for map-based cloning. The dht1 locus was primarily mapped to the long arm of chromosome 4 between the molecular markers L-3 and L-4 (Figure 1I). Further, we utilized 2553 mutant individuals to narrow down the candidate region to a 46-kb genomic region between the markers L-9 and L-49. This region contains seven putative open-reading frames (ORFs) (Figure 1I). By sequencing the 46-kb region, we found a single nucleotide substitution from adenine (A) to thymine (T) in the exon of ORF4 (LOC_Os04g54440), resulting in the substitution of an isoleucine (I) residue by a phenylalanine (F) residue (Figure 1J). To test whether the mutation of LOC_Os04g54440 causes the dht1 defective phenotypes, we introduced the entire genomic region of LOC_Os04g54440 including its flanking sequences into the dht1 mutant. Positive transgenic plants essentially restored the defective phenotypes of dht1, including plant height, tillers, and size of panicles and grains (Figure 1, K–M and Supplemental Figure S3, A and B). In addition, we generated DHT1 knockout mutants using the CRISPR/Cas9 method. The DHT1 knockout mutants displayed similar but more severe defective phenotypes (such as much more tillers) than the dht1 mutant (Supplemental Figure S4, A–F). We also obtained DHT1-RNAi transgenic plants with evident reduction in DHT1 mRNA level and these plants also displayed defective phenotypes similar to the dht1 mutant (Supplemental Figure S5, A–F). Together, these findings verify that LOC_Os04g54440 corresponds to the DHT1 gene.
DHT1 is widely expressed in plant tissues
DHT1 is predicted to encode a 515-amino-acid hnRNP-like protein with two putative RNA recognition motifs (RRMs). Phylogenetic analysis revealed that DHT1 is strictly located in a monocotyledon-specific clade of UBA2 family of hnRNP superfamily (Supplemental Figure S6). Though DHT1 possesses two short highly conserved ribonucleoprotein domains (RNP1 and RNP2) in each RRM (Maris et al., 2005), they also have highly conserved C-terminal (nuclear localization signal) and N-terminal domains that are distinct from other clades of UBA2 family proteins (i.e. AtUBA2a/b, RBP-Q, and RBP-P) (Supplemental Figure S7; Lambermon et al., 2002; Yang et al., 2014b; Tian et al., 2018), implying that UBA2 family proteins may have diverged functions.
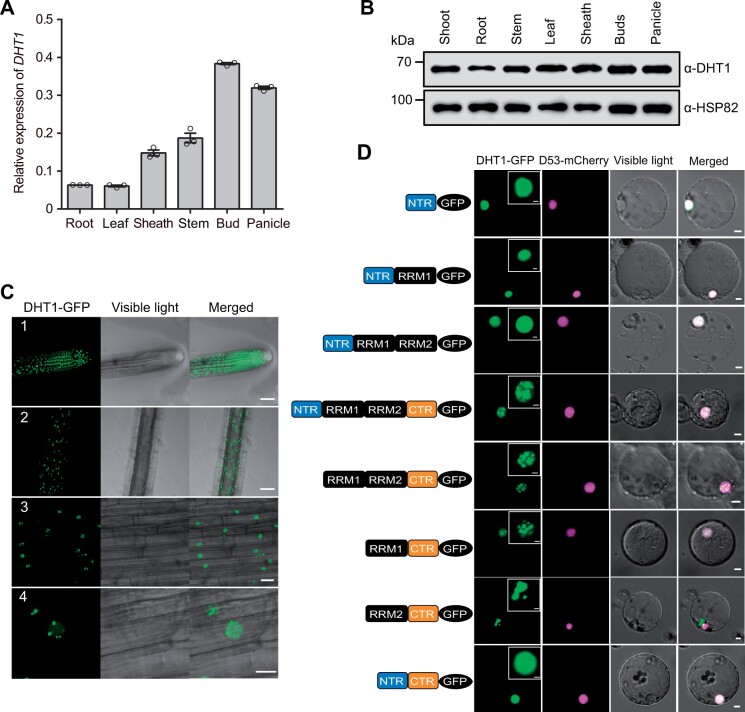
Reverse-transcription quantitative PCR (RT-qPCR) analysis showed that DHT1 was broadly expressed in various plant organs, with preferential expression in the axillary buds and young panicles (Figure 2A). Furthermore, the expression pattern of DHT1 was verified by histochemical staining of the DHT1pro:GUS reporter gene transgenic plants (Supplemental Figure S8, A–G). Additionally, we generated anti-DHT1 specific antibodies using a polypeptide (including amino acids 2–114 of the DHT1 protein) as the antigen. Immunoblot analysis using anti-DHT1-specific antibodies also detected extensive accumulation of the DHT1 protein in various tissues, including shoots of seedling, stems, leaves, buds, and young panicles, corroborating with the pleiotropic phenotype of the dht1 mutant plants (Figure 2B).
Figure 2.
DHT1 gene is widely expressed in plant tissues and the DHT1 protein is localized in nuclear speckles. A, RT-qPCR analysis showing DHT1 transcript levels in different rice tissues. Data are means ± sem (n = 3). B, Immunoblot analysis showing DHT1 protein levels in different tissues. C, Nuclear localization of DHT1-GFP(green fluorescent protein) fusion protein in transgenic rice root cells. Number 1 shows the cell division region in roots; number 2 shows the cell maturation region in roots; numbers 3 and 4 are magnified images of the cell maturation region in roots. Scale bars: 50 μm (1, 2), 20 μm (3), and 10 μm (4). D, The subcellular localization of different domains of DHT1 protein in rice protoplasts. Scale bars, 5 μm. The top right inset images show the magnified green fluorescent portions of DHT1-GFP. Scale bars, 2 μm.
DHT1 co-localizes with SR proteins in nuclear speckles
To determine the subcellular of DHT1 protein, we generated transgenic plants carrying the DHT1-GFP transgene driven by the CaMV 35S promoter. Laser confocal microscopy examination of the root tip cells found that the DHT1-GFP fusion protein was mainly localized in the cell division region of young roots, and exclusively distributed in bright nuclear speckles (Figure 2C). This subcellular localization pattern of DHT1-GFP was also confirmed by transient expression of the DHT1-GFP reporter gene in rice protoplasts (Figure 2D). To further analyze its nuclear localization pattern, we constructed a series of domain deletion mutant versions of DHT1 fused with GFP. Domain deletion analysis revealed that either NTR or RRM1 is sufficient for the nuclear localization of DHT1 and that RRM1 and the C-terminal domain (CTR) are required for nuclear speckle localization of DHT1 (Figure 2D).
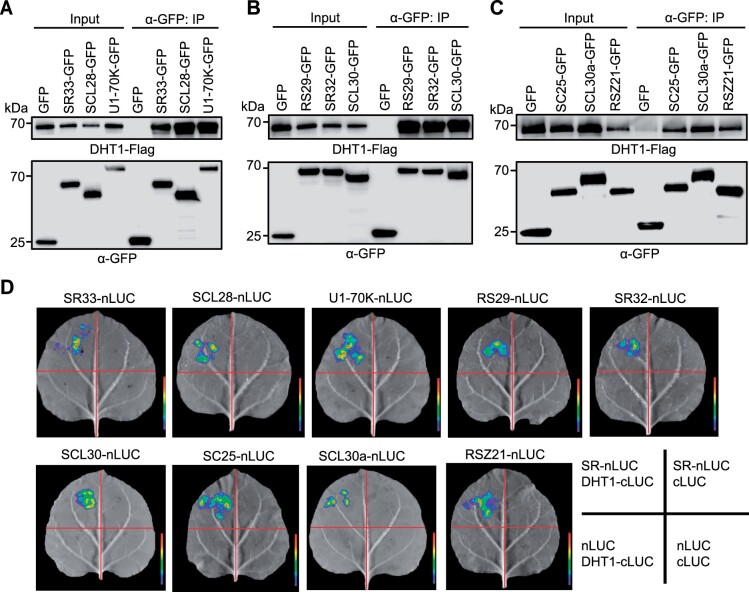
During the assembly of a spliceosome complex, a large number of catalytic and auxiliary proteins, such as snRNPs (U1, U2, U5, and U4/U6 snRNPs) and SR proteins, are recruited to form stable and functional spliceosome complex in nuclear speckles (Rino and Carmo-Fonseca, 2009; Wahl et al., 2009; Mao et al., 2011). To test whether DHT1 co-localizes with SR proteins in rice, DHT1-GFP and rice homologous SR proteins fused with a mCherry fluorescent protein were transiently co-expressed in Nicotiana benthamiana epidermal cells. The results revealed that DHT1-GFP largely co-localized in nuclear speckles with a number of different SR proteins: RS29, RSZ21, SCL28, SCL30a, SCL30, SC25, SR32, and SR33 (Barta et al., 2010; Supplemental Figure S9). In addition, DHT1-GFP is also partially co-localized with U1-70K-mCherry fusion protein (U1-70K, a subunit of U1 snRNP) in nuclear speckles (Supplemental Figure S9). Moreover, our in vitro pull-down assay, co-immunoprecipitation (Co-IP) assay, and firefly luciferase (LUC) complementation imaging assay showed that DHT1 could physically interact with these SR proteins and U1-70K (Figure 3, A–D and Supplemental Figure S10). Together, these results suggest that DHT1 likely acts as a new component of the U1 snRNP and is likely assembled into U1 snRNP spliceosome through interacting with SR proteins and U1-70K.
Figure 3.
DHT1 interacts with SR proteins or U1 snRNP subunit U1-70K. A–C, Co-IP assays in rice protoplasts using anti-GFP antibody show that DHT1 can interact with SR proteins (SR33, SCL28, RS29, SR32, SCL30, SC25, SCL30a, and RSZ21) and U1 snRNP subunit 70K protein (U1-70K). Total proteins were extracted from transformed rice protoplasts co-expressing DHT1-Flag, SRs-GFP and U1-70K-GFP. Immunoprecipitated samples were detected using anti-Flag and anti-GFP antibodies, respectively. D, LUC complementation imaging assay showing the physical interactions between DHT1 with SR proteins (SR33, SCL28, RS29, SR32, SCL30, SC25, SCL30a, and RSZ21) and U1-70K proteins in N. benthamiana epidermal cells.
Impaired stability and RNA-binding activity of the DHT1I232F mutant protein
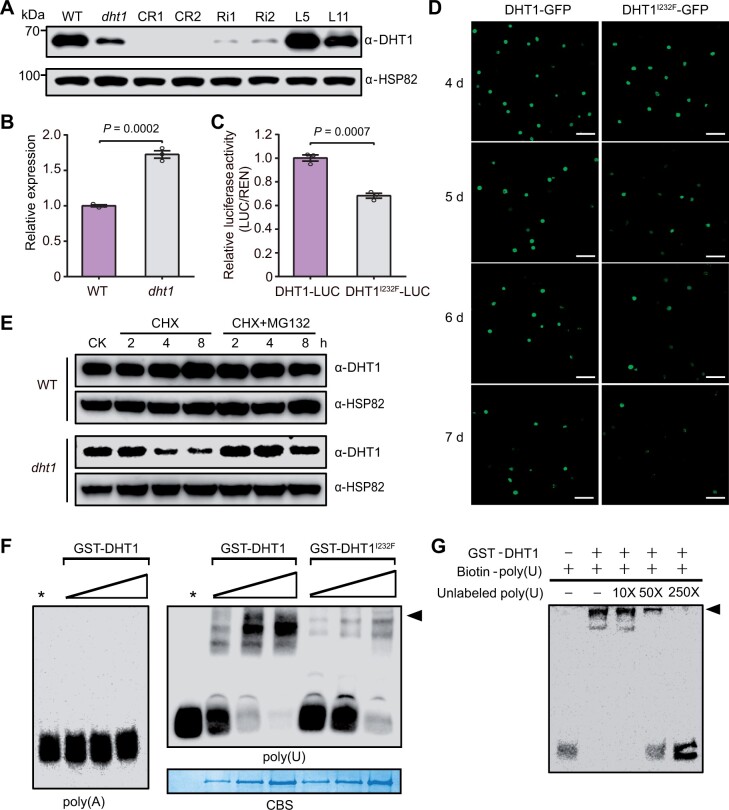
We next compared the gene expression and protein accumulation to explore the underlying mechanisms of dht1 mutant phenotype. We found that the level of DHT1I232F mutant protein was evidently reduced in the dht1 mutants compared with the DHT1 WT protein in WT even though RT-qPCR analysis showed that the mRNA level of dht1 was higher than that of DHT1 (Figure 4, A and B). As expected, the DHT1 proteins in the DHT1 knockout (CR1 and CR2) plants were hardly detectable, and the DHT1 protein levels in DHT1-RNAi plants (Ri1 and Ri2) were significantly reduced compared with WT. The DHT1 protein levels were restored to the WT level in the complemented plants (L5 and L11) (Figure 4A). These observations suggest that I to F amino acid substitution of protein in the dht1 mutant may affect its stability.
Figure 4.
Impaired stability and RNA-binding activity of the DHT1I232F mutant protein. A, Immunoblot analysis showing DHT1 protein levels in WT, dht1 and various transgenic plants. CR1 and CR2 are two independent DHT1 knockout lines; Ri1 and Ri2 are two independent DHT1-RNAi lines; L5 and L11 are two independent complemented transgenic lines. Anti-HSP82 was used as a loading control. B, RT-qPCR analysis of mRNA levels of DHT1 in WT and dht1 plants. Data are means ± sem (n = 3). P-values are from two-sided Student’s t test. C, Relative luciferase activity of DHT1-LUC and DHT1I232F-LUC in the N. benthamiana leaves. Data are means ± sem (n = 3). P-values are from two-sided Student’s t test. D, Confocal scanning images showing the degradation time course of DHT1-GFP and DHT1I232F-GFP fusion proteins in N. benthamiana leaves. Scale bar, 50 μm. E, Immunoblot analysis of DHT1 protein in WT plants and DHT1I232F protein in dht1 plants after the treatment with 3 mM CHX or 3 mM CHX +50 μM MG132. CK represents WT and dht1 plants without treatment. F, In vitro RNA-binding assay showing the binding abilities of GST-DHT1 and GST- DHT1I232F to poly(A30) and poly(U30), with increased protein amounts (0.1, 0.5, and 1 µg). Stars indicate no protein added. Black arrowhead indicates the RNA-protein complex. Coomassie blue staining showing the GST-DHT1 (left three stripes) and GST-DHT1I232F (right three stripes) proteins used for the RNA-binding assay. G, Competitive RNA-EMSA experiment. Unlabeled poly(U30) probes were added into the reaction mixture as the competitive probes with increased concentrations. Black arrowhead indicates the RNA-protein complex.
To further test this notion, we performed a series of biochemical experiments. Luciferase assay showed that relative fluorescence activity of the DHT1I232F-LUC fusion protein was reduced compared with that of DHT1-LUC (Figure 4C). In addition, through transiently expressing DHT1-GFP and DHT1I232F-GFP in tobacco epidermal cells, we found that the fluorescence signals of DHT1I232F-GFP faded much faster than that of DHT1-GFP (Figure 4D). Further, the WT DHT1 protein remained very stable under either the cycloheximide (CHX) or CHX+MG132 treatment, but the level of the DHT1I232F mutant protein rapidly reduced under CHX treatment alone, and degradation of the DHT1I232F mutant protein was partially blocked in the presence of both CHX and MG132, suggesting that DHT1I232F might be subjected to 26S proteasome-mediated degradation (at least partially) (Figure 4E). Taken together, these results support the conclusion that the point mutation in DHT1I232F rendered reduced stability.
Previous studies showed that AtUBA2a and AtUBA2b, the homologs of VfAKIP1 (Li et al., 2002), can interact with UBP1 to bind U-rich sequences in plant 3′-UTR for stabilizing target mRNAs (Lambermon et al., 2002). To test if DHT1 also specifically binds to U-rich sequences, we purified recombinant GST-DHT1 protein to carry out in vitro RNA binding experiments. The results confirmed that DHT1 could bind to poly(U) but not poly(A) (Figure 4F). In addition, the binding ability of GST- DHT1I232F to poly(U) was severely weakened, compared with GST-DHT1 (Figure 4F), indicating that the mutation in DHT1I232F likely attenuates its binding ability to RNAs. Competition assays with different gradients of unlabeled poly(U) further showed that unlabeled poly(U) can weaken the binding ability of GST-DHT1 to labeled poly(U) (Figure 4G), thus confirming that GST-DHT1 can specifically bind to poly(U).
DHT1 regulates pre-mRNA splicing and intron splicing of D14
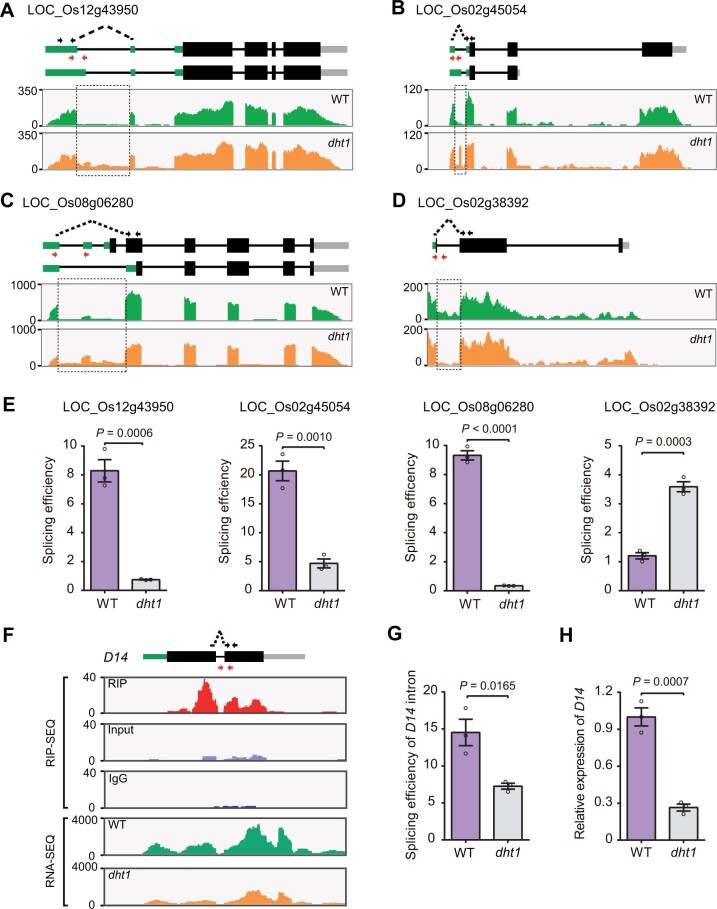
To test whether DHT1 participates in pre-mRNA splicing, we conducted an RNA immunoprecipitation-sequencing (RIP-seq) analysis to identify candidate target transcripts of DHT1. We found many transcripts of snRNAs (i.e. U1 snRNA or U5 snRNA, which are the major components of a spliceosome), in the RIP-seq data (Supplemental Figure S11), further confirming that DHT1 likely acts as a component of U1 snRNP and participates in the assembly of the spliceosome complex during intron splicing. We next conducted an RNA-sequencing (RNA-seq) assay and identified 1,656 potential splicing defects (P-value < 0.05) in the dht1 mutants (Supplemental Data Set S1). Among them, intron retention (IR) events accounted for the majority of splicing defect events in the dht1 mutant compared with WT (Supplemental Figure S12). To further classify the alternative splicing (AS) events in the dht1 mutant, we calculated the proportion of AS events according to their positions in the 5′ UTR, 3′ UTR, and gene coding region (CDS), respectively. We found that the majority of the AS events occurred in the gene coding region (Supplemental Figure S13 and Supplemental Data Set S2). In addition, we randomly selected four defectively spliced genes (LOC_Os12g43950, LOC_Os02g45054, LOC_Os08g06280, and LOC_Os02g38392) for independent verification (Figure 5, A–D). Consistent with the RNA-seq data (Supplemental Figure S14), RT-qPCR analysis showed that the splicing efficiency of LOC_Os12g43950, LOC_Os02g45054, and LOC_Os08g06280 reduced ∼11-, 4-, and 26-fold, respectively, whereas LOC_Os02g38392’s splicing efficiency increased approximately three-fold in the dht1 mutant compared with that of WT (Figure 5E). These results demonstrate that DHT1 indeed plays a general role in pre-mRNA splicing of a large number of genes in rice.
Figure 5.
DHT1 regulates pre-mRNA splicing and intron splicing of D14. A–D, Confirmation of splicing defects between WT and dht1 mutant by RNA-seq. The sequencing read abundance of RNA-seq data is indicated as wiggle plots (green for WT; orange for dht1) in the bottom panel. The genomic structure of gene is shown at the top. Black arrows indicate the primers used to measure the spliced events. The spliced primers crossing exon–exon junctions are represented by black dotted arrows. Red arrows represent the primers used to measure the unspliced events. The dashed rectangle showing locations on the gene where splicing defects occur. E, Corresponding splicing efficiencies of the genes (shown on Figure 5, A–D) in WT and dht1 mutant by RT-qPCR. The splicing efficiency was calculated as the ratio of spliced RNAs to unspliced RNAs, with the primers shown in Figure 5, A–D. F, Profile of DHT1 binding to the transcripts of D14 gene in WT and the abundance of D14 transcript between WT and dht1. The genomic structure of D14 is shown at the top. Black arrows crossing the exon-exon junctions indicate the primers for detecting the spliced events. Red arrows represent the primers for detecting the unspliced events. Wiggle plots of RIP-seq (red for RIP; purple for Input; blue for IgG) and RNA-seq (green for WT; orange for dht1) are shown below the gene structure. The y-axis indicates sequencing read abundance of RIP-seq data or RNA-seq data. The x-axis indicates D14 gene chromosomal position. G, Splicing efficiencies of the D14 gene in the tiller buds of WT and dht1 by RT-qPCR validation. The splicing efficiency was calculated as the ratio of spliced D14 RNA to the level of unspliced D14 RNA. H, RT-qPCR analysis showing D14 mRNA levels in the tiller buds of WT and dht1. Data in (E, G, and H) are means ± sem (n = 3). P-values are from two-sided Student’s t test.
Further, we analyzed the overlap between 3,859 DHT1 bound target genes (Supplemental Data Set S3) and 1,285 genes with altered splicing events in the dht1 mutant (excluding repeated mRNAs with AS events occurring in the same gene and mRNAs without a login number in the MSU database) (Supplemental Data Set S1) and identified 116 overlapping target transcripts (Supplemental Figure S15). One-tailed hypergeometric test (https://stattrek.com/online-calculator/hypergeometric.aspx) showed a significant enrichment of the 116 overlapping genes against the total 55,801 loci in rice (http://rice.uga.edu/) (P-value = 0.0021, n ≥ 116). Thus, the 116 genes could be viewed as viable targets alternatively spliced by DHT1.
Interestingly, we found significant DHT1-binding peaks in exons flanking the sole intron of the D14 gene transcript (Figure 5F and Supplemental Data Set S3), which encodes the SL receptor (Yao et al., 2016). This finding points to D14 as a potential target of DHT1. Although the RNA-seq data and RT-qPCR data from 3-week-old plants showed that IR in the D14 transcript in dht1 was not evidently different from that in WT (Figure 5F and Supplemental Figure S16), the splicing efficiency of the D14 intron in the tiller buds of dht1 was significantly reduced compared with that in WT (Figure 5G and Supplemental Figure S17). Consistent with this finding, RT-qPCR analysis showed that the transcript level of D14 was obviously reduced in the tiller buds of dht1 mutant compared with WT (Figure 5H). We speculated that retention of the intron in D14 transcript may trigger the nonsense-mediated decay (NMD) pathway due to introduction of a premature stop codon in its transcript (Supplemental Figure S18; Shaul, 2015), thus leading to accelerated degradation of the D14 mRNA. Together, these results showed that both splicing of D14 pre-mRNA and stability of D14 mRNA are affected in the dht1 mutant.
DHT1 regulates rice tillering through mediating the SL signaling pathway
Given the above observations, we hypothesized that the SL-deficient-like phenotype of dht1 might be (at least partially) caused by defects in proper splicing of D14 pre-mRNA and stability of D14 mRNA. Hence, we first measured the content of SLs in root exudates and found that the exudated amount of 2′-epi-5-deoxystrigol (epi-5DS) from dht1 was approximately five times of that from WT (Figure 6A). Second, exogenous GR24 (an analog of SL) treatment showed that dht1 had reduced sensitivity to GR24, a synthetic SL analog (Figure 6B). Thirdly, RT-qPCR analysis showed that although the expression of DHT1 was not evidently changed between the d53 mutant and its background parent Norin 8, it was obviously decreased in all five SL biosynthesis or signaling-related mutants (d27, d17, d10, d3, and d14) compared with their WT (Ishikawa et al., 2005; Zou et al., 2006; Arite et al., 2007, 2009; Lin et al., 2009; Zhou et al., 2013; Figure 6C), and the DHT1 expression was slightly upregulated by GR24 treatment (Supplemental Figure S19), implying that DHT1 expression is positively regulated by SL signaling. These results suggest that DHT1 likely participates in SL signaling through regulating D14 pre-mRNA splicing.
Figure 6.
DHT1 regulates SL signaling. A, Comparison of epi-5DS levels in WT and dht1 root exudates. g FW is indicated as per gram fresh weight. B, Response of tiller buds in WT, dht1 and d14 to 1 μM GR24 treatment. Red arrows indicate the elongated tiller buds. Scale bars, 2 cm. C, Expression levels of DHT1 in five recessive d mutants (d27, d17, d10, d3, and d14) with Kitaake background and the expression level of DHT1 in the dominant d53 mutant with Norin 8 background. D, Immunoblot showing lower D14 protein levels in the tiller buds of dht1 compared with that of WT plants. E, Immunoblot showing higher D53 protein levels in the tiller buds of dht1 and DHT1 knockout plants (CR1 and CR2) compared with these of WT plants. F, Immunoblot showing D53 protein levels in WT, CR1 and d14 mutant plants after 3 h treatment with 5 µM GR24. G, Tiller number of WT, dht1 and dht1D14-3xFlag overexpression transgenic plants at the mature stage. H, Tiller number of WT, dht1 and dht1D53-RNAi transgenic plants at the mature stage. I, Immunoblot showing D14 and D53 protein levels in the tiller buds of WT, dht1, and dht1D14-3xFlag overexpression transgenic plants. J, Immunoblot showing D53 levels in WT, dht1, and dht1D53-RNAi transgenic plants. K, Tiller number of WT, dht1 and dht1D53-CRISPR transgenic plants at the mature stage. L, Phenotypes of WT, dht1, dht1D14-3xFlag, dht1D53-RNAi, and dht1D53-CRISPR transgenic plants at the mature stage. Scale bars, 5 cm. Data are means ± sem (n = 3) and P-values are from two-sided Student’s t test (A and C). Data are means ± sem (n = 7) and P-values are from one-way ANOVA test with Tukey's correction (G, H, and K). Anti-HSP82 was used as a loading control in (D, E, F, I, and J).
To test the above hypothesis, we conducted immunoblot analysis to compare protein accumulation of D14 and D53 in the dht1 mutant and WT. The D14 protein level in the dht1 mutant was markedly reduced, while the accumulation of the SL signaling central repressor protein D53 was significantly higher in both the dht1 mutant and DHT1-CRISPR knockout mutants (Figure 6, D and E). Further, we found that GR24 treatment-triggered degradation of D53 protein was largely abolished in the DHT1-knockout mutant plants and d14 mutant, compared with WT (Figure 6F). Taken together, these results suggest that DHT1 represses rice tillering likely by stabilizing D14 expression/splicing to ensure proper SL signaling.
To further explore the genetic relationship between DHT1 and D14, we overexpressed D14 in the dht1 mutant background, and found that increased expression of D14 in the dht1 background effectively restored the tiller number phenotype (Figure 6, G, I, and L). To investigate the genetic relationship between DHT1 and D53, we generated transgenic lines with reduced D53 expression by RNAi or D53 knockout by CRISPR/Cas9 technology (Supplemental Figure S20) in the dht1 mutant background. The dht1D53-RNAi or dht1D53-CRISPR transgenic lines exhibited largely restored tiller number phenotype (Figure 6, H, J–L). These results suggest that both D14 and D53 act genetically downstream of DHT1 in regulating rice tillering.
Discussion
In this study, we found that DHT1 encodes a member of a previously unrecognized monocotyledon-specific clade of UBA2 family of hnRNP proteins, containing two RRM domains (Supplemental Figures S6 and S7). Previous studies showed that AKIP1, a DHT1 homolog in Vicia faba (Supplemental Figure S6), interacts with ABA-activated AAPK to regulate mRNA stability of the dehydration proteins (Li et al., 2002); whereas Arabidopsis AtUBA2, another DHT1 homolog in the same clade of UBA2 family as AKIP1 (Supplemental Figure S6), acts as an interacting factor of UBP1 to recognize U-rich sequences in plant 3′ UTRs for stabilizing target mRNAs in the nucleus (Lambermon et al., 2002). In this study, we accumulated several lines of evidence supporting that DHT1 acts as a component of the spliceosome and is required for proper pre-mRNA splicing of many genes including D14. First, we found that it physically interacts and co-localizes with a number of SR proteins and U1-70K (Figure 3 and Supplemental Figures S9 and S10); second, we showed that it can specifically bind to poly(U) but not poly(A) (Figure 4F), suggesting that DHT1 likely acts as a component of the U1 snRNP and is likely assembled into U1 snRNP spliceosome through interacting with SR proteins and U1-70K; third, we found that DHT1 binds to a large number of transcripts, including the pre-mRNA of D14 (Supplemental Data Set S3); fourth, the splicing efficiency of D14 pre-mRNA in the tiller buds of dht1 mutant was reduced compared with that of WT (Figure 5G and Supplemental Figure S17). Therefore, our findings provide insights into the functional diversification of the UBA2 family of proteins.
Moreover, our results showed that the transcripts of the D14 gene are reduced in dht1 compared with WT (Figure 5H) and that the DHT1I232F mutant protein possesses reduced binding ability to U-rich sequence (Figure 4F) and DHT1 directly binds to the U-rich sequences in 3′ UTR of D14 mRNA (Supplemental Figure S21). The above results imply that DHT1 may also directly stabilize its mRNA abundance by binding 3′ UTR of D14 mRNA. It is also worth noting that in the dht1 mutant, the intron-retained D14 mRNA contains a premature stop codon (Supplemental Figure S18). It is well known that intron retained transcripts harboring premature termination codon are generally transported into the cytoplasm for degradation by NMD, contributing significantly to downregulation of transcript levels in order to maintain transcriptome homeostasis (Drechsel et al., 2013; Braunschweig et al., 2014). Recent studies also showed that some intron-containing transcripts could be remained in the nucleus to escape NMD (Gohring et al., 2014; Jia et al., 2020) and the nucleus-localized intron-containing transcripts might be subject to induced degradation by a nuclear surveillance machinery, leading to downregulation of gene expression (Yap et al., 2012; Pimentel et al., 2016; Wong et al., 2016). Thus, the reduced levels of D14 mRNA in the dht1 mutant might also be caused by accelerated degradation of D14 mRNA by the NMD pathway or retention of unspliced D14 pre-mRNA in the nucleus. Further investigation is required to clarify the detailed mechanisms.
Rice tillering is an important factor that determines plant architecture and grain yield. In rice, D14 acts as a signal receptor for SL, which regulates the ubiquitination and proteasome degradation of the central repressor protein D53, thereby unleashing SL signaling and suppressing rice tillering (Jiang et al., 2013; Zhou et al., 2013; Zhao et al., 2015). In this study, we found that splicing efficiency of D14 intron decreased significantly and the transcript level of D14 was downregulated in the tiller buds of dht1 mutant (Figure 5, G and H); correspondingly, the tiller buds of dht1 mutant had reduced level of D14 protein and increased accumulation of D53 protein, thus causing more tillers in the dht1 mutant (Figures 1, D, G, and H and 6, D and E). In addition, overexpression of D14 or reduction of D53 protein in the dht1 mutant background can largely restore the tiller defects of the dht1 mutant (Figure 6, G–L). These results demonstrate that DHT1 represses rice tillering by regulating the intron splicing of D14 pre-mRNA, and thus affecting SL signaling (Figure 7). Our findings establish a functional link between posttranscriptional splicing of D14 pre-mRNA and SL signaling, which may offer new strategies for improving agronomic traits of crops through fine-tuning posttranscriptional regulation of gene expression through molecular breeding.
Figure 7.

Proposed working model for DHT1 influence on rice tillering through regulating D14 intron splicing. During the splicing of the pre-mRNA, DHT1 assembles with U1 snRNP and SR proteins to form a spliceosome to efficiently splice D14 intron for its effective translation, which can provide a sufficient amount of D14 protein for efficient SL signaling to induce the degradation of the SL signaling repressor D53 by the SCFD3-D14 E3 ubiquitin ligase complex, thus inhibiting rice tillering.
Interestingly, we found that some dicots, such as Arabidopsis thaliana, Brassica napus, Camelina sativa, and Solanum tuberosum, have intron-less D14 homologous genes while most dicots still have intron-containing D14 homologous genes (Supplemental Figure S22). In addition, close DHT1 homologous genes in dicots are not grouped into the monocotyledon-specific DHT1 clade (Supplemental Figure S23). These results suggest that most dicots likely recruit a class of unidentified hnRNP-like proteins to regulate splicing and expression of their D14-like genes, whereas hnRNP-like proteins are likely not required for splicing intron-less D14-like genes in some dicots such as A.thaliana, B.napus, C.Sativa, and S.tuberosum.
It is worth pointing out that besides D14 pre-mRNA, DHT1 also binds to a large number of transcripts that play important roles in plant growth and development (Supplemental Data Set S3), such as gibberellin synthesis genes OsGA20ox2 (Sasaki et al., 2002), cytokinin synthesis genes LOG (Kurakawa et al., 2007), cytokinin signaling genes OsRR6 (Ito and Kurata, 2006), and auxin signaling genes OsAUX1 and OsARF19 (Zhang et al., 2016; Giri et al., 2018). In addition, DHT1 can also bind to transcripts of OsAGO1d that regulates miRNA processes (Wu et al., 2009), the arginine methyltransferase gene OsPRMT1 responsible for histone methylation (Ahmad et al., 2011), the cell wall biosynthesis gene OsCSLD4 (Li et al., 2009), and so on. These observations suggest that DHT1 likely regulates multiple cellular processes by modulating the expression and pre-mRNA splicing of key genes in multiple signaling pathways to affect plant growth and development. Interestingly, some DHT1-binding targets are related to salt, drought and heat stress, such as OsHSP17.0, OsEIL2, OsMSR2, and OsNAC6 (Nakashima et al., 2007; Xu et al., 2011; Zou et al., 2012; Yang et al., 2015), and some related genes are involved in rice disease resistance, such as OsSAPK2, OsWRKY13, FucT and Rh3 (Chern et al., 2012; Xu et al., 2013; Cheng et al., 2015; Harmoko et al., 2016). Further studies are required to elucidate the multifaceted role of DHT1 in regulating plant development and responses to various stresses.
Materials and methods
Plant materials and growth conditions
The mutant dht1 was obtained from a tissue culture-derived population, which the plants population originated from tissue culture and have higher frequencies of mutation (Phillips et al., 1994). WT control was the japonica cultivar Kitaake. The d mutants including d14, d3, d27, d17, and d10 mutants in the Kitaake background used in the study were isolated in our laboratory and their mutation sites are shown in Supplemental Figure S24. The d53 mutant with Norin 8 as the background was described previously (Zhou et al., 2013). All mutants, WT and transgenic plants were cultivated in the experimental fields at the Chinese Academy of Agricultural Science (Beijing, 116°130′E, 39°54′N) under the natural growing seasons. For RT-qPCR assays, GR24 treatment, SL analyses and Co-IP assays, the seedling of WT and mutants were grown in climate chambers (HP1500GS; Ruihua, Wuhan, China) at 70% humidity, under long-day conditions with a photocycle of 14-h light (30°C) and 10-h darkness (25°C). Light was provided by fluorescent white-light tubes (400–700 nm, 250 μmol m−2 s−1).
Rice hydroponic culture and SL analysis
Rice seeds were surface-sterilized and incubated at 30°C in the dark for 2 days. Germinating seeds were transferred into hydropic culture solution for 5 days. For GR24 treatment, 1-week-old seedlings were transferred to a plastic pot containing hydropic culture solution (1 L) with or without GR24 (1 μM) and grown for 6 weeks. The hydropic culture solution was renewed every 3 days, with fresh solution containing GR24. For SL analysis, 1-week-old seedlings were transferred into hydropic culture medium without Pi and grown for an additional 14 days. Levels of epi-5DS in rice root exudates were measured using the method as described in Zhou et al. (2013).
Map-based cloning of DHT1
For cloning the DHT1 gene, we crossed the mutant dht1 with a polymorphic japonica variety IRAT129, and selected 2,553 mutant individuals from the F2 population for gene mapping. The corresponding indel markers and CAPS markers are listed in Supplemental Table S1. To confirm the mutant site, the cDNA of the candidate gene DHT1 was amplified from dht1 and WT plants with specific primers and sequencing confirmed.
Vector construction and rice transformation
To generate the complementation construct, a 7.5-kb genomic DNA fragment, consisting of 2.3-kb promoter, the entire coding sequence of the DHT1 gene, and 3.5-kb downstream region, was PCR-amplified from the genomic DNA of Kitaake using primers DHT1-1305SEQ-F and DHT1-1305SEQ-R, and inserted into the Sac I and Bam HI site of the binary vector pCAMBIA1305.1.
To generate the DHT1-RNAi construct, both sense and antisense versions of a 241-bp specific fragment from the coding region of the DHT1 gene were amplified with the primer pairs DHT1-RNAi-sense and DHT1-RNAi-antisense, sequentially cloned into the vector pCUbi1390-ΔFAD2. Likewise, the 475-bp specific fragment used to construct the D53-RNAi vector was amplified with primers shown in Supplemental Table S2 and sequentially inserted into the vector pCUbi1390-ΔFAD2.
To generate the CRISPR/Cas9 DNA knockout constructs, pCAS9-DHT1, pCAS9-D53, 20-bp gene-specific spacer sequence of the target genes was cloned into the vector CRISPR-Cas9 according to a previously reported method (Miao et al., 2013).
To generate the overexpression vector pCUbi1390-D14, the coding region of D14 was amplified using the primers shown in Supplemental Table S2, and the PCR product was cloned into the binary vector pCUBI1390 with the In-Fusion Advantage PCR Cloning Kit (Clontech, Mountain View, California, USA).
All these constructs were introduced into Kitaake or the dht1 mutant by Agrobacterium tumefaciens-mediated transformation (Hiei et al., 1994).
RNA extraction and RT-qPCR
Total RNA from roots, leaves, sheaths, stems, buds, and young panicles were isolated using a ZR Plant RNA MiniPrep Kit (ZYMO Research, Irvine, California, USA) and treated with DNaseI (TaKaRa, Shiga, Japan) to remove the genomic DNA according to the manufacturer’s protocol. First-strand cDNA was reverse transcribed from 1 μg of total RNA using a QuantiTect reverse transcription kit (Qiagen, Hilden, Germany) according to the manufacturer’s instruction. RT-qPCR was performed with an SYBR premix Ex Taq Kit (TaKaRa) on an ABI prism 7500 Real-Time PCR System according to the operation manual. The rice UBIQUITIN gene was used as an internal control for data normalization. Three biological replicates for each sample were analyzed. ACTIN gene was used as an internal control for RT-PCR. The primers for RT-qPCR and RT-PCR analysis are listed in Supplemental Table S3.
GUS staining
For analyzing the expression pattern of DHT1, the 2.8-kilobase promoter fragment was amplified using the primers shown in Supplemental Table S2 and inserted into the Eco RI and Nco I sites of the binary vector pCAMBIA1305.1 to generate the plasmid DHT1pro:GUS. The plasmid was transformed into Kitaake by A.tumefaciens-mediated transformation. Different tissues of the transgenic plants at different developmental stages were collected. Histochemical GUS staining was performed according to a previous report (Jefferson et al., 1987).
Antibody preparation and immunoblot analysis
The coding region for 2-114 aa of DHT1 was amplified with the specific primers shown in Supplemental Table S2. The PCR product was cloned into the Bam HI and Eco RI sites of the pET28a vector. The plasmid was expressed in BMRosetta (DE3) cells and affinity purified. Purified His-DHT1 fusion protein was injected into rabbits to produce polyclonal antibodies of DHT1. 1:1,000 dilution of the DHT1 antibody was used for immunoblotting. For detection of the D14 protein, the coding region of D14 was amplified using the primers shown in Supplemental Table S2 and the PCR product was cloned into the pET28a vector. The plasmid was expressed in BMRosetta (DE3) cells and affinity purified. Purified His-D14 fusion protein was injected into rabbits to produce polyclonal antibody of D14. 1:1,000 dilution of the D14 antibody was used for immunoblotting. The anti-D53 antibody was previously described by Zhou et al. (2013). The loading control used is anti-HSP82 (Beijing Protein Innovation, Beijing, China; at 1:5,000 dilution).
For immunoblot analysis, the total proteins were added with 6× extracting buffer (0.1 mM MG132, 1× loading buffer, 1× PBS, 1:100 protease inhibitor cocktail [Roche, Basel, Switzerland]), and centrifuged for 30 min at 18,407 g. The supernatant was added with 5× SDS loading buffer and boiled at 95°C for 10 min. The extracted protein was separated on SDS-PAGE gels and transferred electrophoretically onto nitrocellulose filter membrane. The membrane was incubated with the purified antibodies and visualized with enhanced chemiluminescence reagent (GE Healthcare, Chicago, Illinois, USA).
Subcellular localization of DHT1
For analysis of the subcellular location of the DHT1 protein in plants, the coding region of the DHT1 gene was amplified using primers shown in Supplemental Table S2, and the PCR product was cloned into the Spe I and Bam HI sites of the vector pCAMBIA1305-GFP to generate the plasmid p35S: DHT1-GFP, which was transformed into Kitaake by Agrobacterium-mediated method. GFP fluorescence signal in young roots of transgenic plants was detected with a Zeiss LSM880 confocal laser scanning microscope.
In addition, the full-length coding sequence of DHT1 and truncated cDNA were amplified from Kitaake by PCR using corresponding primers shown in Supplemental Table S2. Subsequently, these fragments were cloned into the Spe I and Xba I sites of the vector PAN580 to generate plasmids that their C-termini were fused to the GFP reporter gene under the control of the CaMV 35S promoter. All of the plasmids were transiently co-expressed into rice protoplasts with the nuclear marker D53-mCherry constructs following the method described previously (Zhang et al., 2011). The fluorescence signals were observed using a Zeiss LSM880 confocal laser scanning microscope.
Transient expression assays in N. benthamiana leaves
For luciferase activity assay, the coding sequences of DHT1 and dht1 were amplified from Kitaake and dht1 mutant plants using DHT1-LUC-F and DHT1-LUC-R, then cloned into Green II 0800-LUC under the control of the CaMV 35S promoter to generate the plasmids DHT1-LUC and DHT1I232F-LUC, respectively. The Renilla luciferase (REN) under the control of the CaMV 35S promoter in Green II 0800-LUC vector was used as the internal control. The plasmid DHT1-LUC and DHT1I232F-LUC were co-infiltrated with Green II 0800-LUC vector into N. benthamiana leaves by A.tumefaciens-mediated transformation, respectively. At 3 days after infiltration, the total proteins were extracted with lysis buffer (50 mM Tris-MES, PH 8.0, 0.5 M Sucrose, 1 mM MgCl2, 10 mM EDTA, 5 mM DTT). Activities of LUC and REN were measured with a Dual-Luciferase Assay System (Promega, Madison, Wisconsin, USA) according to the manufacturer’s protocol. The DHT1- LUC activity and DHT1I232F-LUC activity were calculated as the ratio of LUC/REN activity.
For subcellular localization, the coding sequences of RSZ21, SCL30a, SCL28, SCL30, SC25, SR33, RS29, SR32, and U1-70K were amplified from Kitaake by PCR using corresponding primers shown in Supplemental Table S2, and then inserted into the vector pCAMBIA 1305-mCherry. The coding sequences of DHT1 and dht1 were amplified from Kitaake and dht1 mutant plants using the same primers DHT1-1305GFP-F and DHT1-1305GFP-R and cloned into the vector pCAMBIA 1305-GFP to generate the plasmid p35S: DHT1-GFP and p35S: DHT1I232F-GFP, respectively. The resulting mCherry plasmids and DHT1-GFP were separately cotransfected into Agrobacterium strain EHA105 with p19 strain into young N. benthamiana leaves using an injection syringe. The plasmids DHT1-GFP and DHT1I232F -GFP were transferred separately into N. benthamiana leaves as described above. Two days after infiltration, the fluorescence signals were observed and imaged using a Zeiss LSM880 confocal laser scanning microscope for several days.
The LUC complementation imaging assay was performed as previously described (Hua et al., 2012). The DHT1 coding sequence was fused to the C-terminus of LUC to generate the plasmid DHT1-cLUC. Similarly, the coding sequences of SR proteins and U1-70K protein were fused to the N-fragment of LUC to generate the plasmids SR-nLUC and U1-70K-nLUC, respectively. DHT1-cLUC with SR-nLUC and U1-70K-nLUC were co-infiltrated into N. benthamiana leaves via Agrobacterium-mediated transformation. Two days after infiltration, the leaves were applied with 1 mM luciferin, and kept in darkness for several minutes. Subsequently, the fluorescence signals were observed with Plant Molecular Imaging System in vivo. All primers involved are shown in Supplemental Table S2.
Phylogenetic analysis
The homologous sequences of DHT1 in rice (O.sativa), A.thaliana, human, and other plants were downloaded from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) and the database searches (http://plants. ensembl.org/) using the BLASTP program. Multiple sequence alignment of these proteins was performed using the ClustalW program (Supplemental File S1). The phylogenetic tree was constructed using the neighbor-joining method with 1,000 bootstrap replicates (MEGA version 5 software). Amino acid sequences of DHT1 homologous proteins were aligned and displayed using the DNAMAN software.
RNA electrophoretic mobility shift assay
To generate the recombinant expression protein vector, the coding region of the DHT1 gene was amplified from the WT Kitaake with primers shown in Supplemental Table S2, and the coding region of the mutant dht1 gene was amplified from the mutant plants with the same primers. The PCR products were cloned into the Eco RI and Sal I sites of the vector pGEX-4T-1 to generate glutathione S-transferase-tagged recombinant protein constructs, GST-DHT1 and GST-DHT1I232F. These constructs were transformed into BMRosetta (DE3) cells, propagated in a 37°C incubator. The expression of GST-DHT1 and GST-DHT1I232F proteins were induced at 16°C for 16 h in the presence of 0.5 mM isopropyl-β-D-thiogalactoside. Soluble recombinant proteins were affinity purified using BeaverBeads GSH (Beaver, Waltham, Massachusetts, USA) according to the manufacturer’s protocol. RNA oligoribonucleotide probes (U30, A30, D14-3’UTR-probe-1, -2, and -3) were synthesized and labeled with biotin at the 3′ end (Bioneer, Shanghai, China). The RNA electrophoretic mobility shift assay (RNA-EMSA) was carried out with the LightShift Chemiluminescent RNA EMSA Kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA). For the competitive RNA EMSA, the unlabeled poly(U30) probes were added into the reaction mixture with increasing gradient concentrations. RNA-Probe sequences are shown in Supplemental Table S4.
In vitro pull-down assays
To prepare the recombinant protein expression vectors, the coding region of the DHT1 gene was cloned into pMAL-c2x; CDSs of different SR protein-coding genes and U1-70K were cloned into pGEX-4T-1. These constructs were transformed into the Escherichia coli strain Rosetta (DE3), propagated in a 37°C incubator and induced at 16°C for 16 h in the presence of 0.2 mM isopropyl-β-D-thiogalactoside. Roughly equal amounts of purified GST and GST-SR proteins and GST-U1-70K were affixed to BeaverBeads GSH (Beaver) and then incubated with purified MBP-DHT1 at 4°C for 2 h in 1 mL of binding buffer (1× PBS, 0.2% Triton X-100, and 1:100 protease inhibitor cocktail [Roche]) and washed five times with binding buffer. GST fusion proteins were detected by the HRP-conjugated anti-GST antibody (PM013-7; MBL, 1:5,000 dilution) and MBP-DHT1 was detected by a monoclonal anti-MBP antibody (M091-3; MBL, 1:10,000 dilution). All primers are shown in Supplemental Table S2.
In vivo Co-IP assay
The DHT1 coding sequence was amplified using primers DHT1-FLAG-F and DHT1-FLAG-R, cloned into the vector pCAMBIA1300-FLAG to generate the plasmid DHT1-Flag. The coding sequences of RSZ21, SCL30a, SCL28, SCL30, SC25, SR33, RS29, SR32, and U1-70K were amplified using primers shown in Supplemental Table S2 and cloned into the vector PAN580 to generate GFP fusion constructs. The plasmid DHT1-Flag was co-transferred into rice protoplasts with these SRPs-GFP and empty vector PAN580, respectively, as described above. The Co-IP assay was performed as previously reported (Yang et al., 2014a), with slight modifications. The second day after transformation, the total proteins were extracted using lysis buffer (50 mM Tris–MES, pH 8.0, 0.5 M Sucrose, 1 mM MgCl2, 10 mM EDTA, 5 mM DTT), and centrifuged at 13,000 g for 20 min at 4°C. An aliquot of 20 μL GFP magnetic Beads were added to the protein supernatant, incubated at 4°C for 1 h. Then, the beads were washed five times with 1 mL lysis buffer and boiled in SDS loading buffer at 100°C for 10 min. Sample proteins were separated on a 10% SDS-PAGE gel and detected by immunoblotting using anti-Flag (M185-7; MBL, 1:5,000 dilution) and anti-GFP antibodies (11814460001; Roche, 1:5,000 dilution), respectively.
RNA-seq and RNA-seq data analysis
Total RNAs were extracted from 3-week-old seedlings of WT and dht1 for each of two biological replicates using TRIzol (Invitrogen) and treated with DNase I to remove the genomic DNA according to the user manual. mRNA from 5 µg of total RNA was enriched by magnetic beads with Oligo (dT). The RNA-seq library with unique molecular identifier (UMI) was prepared using KC-DigitalTM Stranded mRNA Library Prep Kit for Illumina® (Catalog NO. DR08502, Wuhan Seqhealth Co., Ltd., Wuhan, China) followed the manufacturer’s protocol. High-throughput sequencing was performed on an Illumina Novaseq 6000 instrument with 150-bp paired-ends reads.
After trimming the adaptor sequence and filtering low-quality reads by Trimmonatic (version 0.36) (Bolger et al., 2014), average ∼44 million clean reads from WT sample and average ∼42 million clean reads from dht1 sample were obtained, respectively. Approximately 97% of clean reads after deduplication using UMI soft (version 1.0) could be mapped to the rice reference genome (http://rice.plantbiology.msu.edu/analyses_search_locus.shtml) using STAR software (version 2.5.3a) (Dobin et al., 2013; Shugay et al., 2014; Supplemental Table S5). Only uniquely mapped reads were used for subsequent analysis. The expression of each transcript from each sample was calculated by counting the number of mapped reads using featureCounts (version 3.2.5) (Liao et al., 2014) and normalized to Reads per Kilobase per Million reads (RPKM) referring to a previously reported method (Mortazavi et al., 2008). The different AS events were analyzed using rMATS software (version 3.2.5) (Shen et al., 2014). The AS events with P-value < 0.05 were defined as differentially spliced between WT and dht1.
RIP-seq and sequence data analysis
Three-week-old WT seedlings of two biological replicates were harvested, ground into powder in liquid nitrogen, and the obtained powder was lysed. The 10% supernatant of the lysed samples was used to extract RNA with TRIzol reagent (Invitrogen) for preparing input RNA and the 80% remaining supernatant was used for RNA-protein complexes IP using 5 µg anti-DHT1 antibodies and named “IP.” And the 10% remaining supernatant was added with 3 µg anti-IgG antibody (Cell Signaling Technology, Danvers, Massachusetts, USA) as a negative control and named “IgG.” After overnight incubation (on a slowly rotating platform) at 4°C, appropriate amount of protein A magnetic beads were added to the incubation solution. After 1 h, the beads were washed three times and then the immunoprecipitated RNAs were extracted using TRIzol reagent (Invitrogen), as IgG or IP RNA. RNA integrity was detected using agarose gel electrophoresis. RNA was quantified by Qubit version 3.0 with Qubit RNA Broad Range Assay kit (Life Technologies Carlsbad, California, USA). The enriched RNA from RIP assay was directly amplified for library preparation. The RIP-seq libraries with UMI were constructed by using KC-DigitalTM Stranded mRNA Library Prep Kit for Illumina® (Catalog No. DR08502, Wuhan Seqhealth Co., Ltd. China) following the manufacturer’s instruction. High-throughput sequencing was performed on an Illumina Novaseq 6000 instrument with 150-bp paired-ends reads.
After quality control-filtered and deduplication using Trimmonatic (version 0.36) and UMI soft (version.1.0) (Bolger et al., 2014; Shugay et al., 2014), average of ∼61 million clean reads were generated from two biological replicates of IP and average of ∼50 million reads of Input were obtained. Approximately 83% high-quality clean reads were mapped to the rice reference genome (http://rice.plantbiology.msu.edu/analyses_search_locus.shtml) using STAR software (version 2.5.3a) (Dobin et al., 2013; Supplemental Table S5). Only uniquely mapped reads were used for subsequent analysis. The expression of transcripts bound by DHT1 was calculated by counting the number of mapped reads and normalized to RPKM. The putative RIP target transcripts were identified as significantly enriched using edgeR (version 3.12.1) (Robinson et al., 2010; McCarthy et al., 2012) (a log2 fold change >1.0, P-value < 0.05) in each of the RIP-seq data compared with input RNA. The sequence profiles showing DHT1 binding sites were visualized using IGV.
Accession numbers
The RNA-seq and RIP-seq data of this article have been deposited in the National Center for Biotechnology Information Sequence Read Archive under accession numbers PRJNA804398 and PRJNA804403, respectively. Sequence from this study can be downloaded from the rice genome annotation project (http://rice.plantbiology.msu.edu/) with the following accession numbers: DHT1, LOC_Os04g54440; D14, LOC_Os03g10620; D53, LOC_Os11g01330; RSZ21, LOC_Os02g54770; SCL30a, LOC_Os02g15310; SCL28, LOC_Os03g24890; SCL30, LOC_Os12g38430; SC25, LOC_Os03g27030; SR33, LOC_Os07g47630; RS29, LOC_Os04g02870; SR32, LOC_Os03g22380; U1-70K, LOC_Os10g02630; Ubiquitin, LOC_Os01g22490; and ACTIN, LOC_Os03g50885.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Reduced cell size of the dht1 mutant stems.
Supplemental Figure S2. Other phenotypes of the dht1 mutant.
Supplemental Figure S3. Panicle and grain phenotypes of WT, dht1, and two complemented transgenic lines.
Supplemental Figure S4. Phenotypes of the DHT1-CRISPR knockout plants.
Supplemental Figure S5. Phenotypes of DHT1-RNAi lines.
Supplemental Figure S6. Phylogenetic analysis of plant UBA2 family and human hnRNP A/B.
Supplemental Figure S7. Protein sequence alignment of dht1, DHT1, and its orthologs.
Supplemental Figure S8. GUS-staining of the DHT1pro:GUS transgenic reporter plants.
Supplemental Figure S9. Co-localization of DHT1 with SR proteins or U1-70k.
Supplemental Figure S10. In vitro pull-down assay of DHT1 with SR protein and U1-70K.
Supplemental Figure S11. DHT1 binds to transcripts of snRNA genes.
Supplemental Figure S12. Statistics of splicing events in dht1 mutant.
Supplemental Figure S13. AS event distribution in dht1 genome.
Supplemental Figure S14. Histograms of splicing efficiency from RNA-seq.
Supplemental Figure S15. Venn diagram showing the overlap between RIP-seq and RNA-seq results.
Supplemental Figure S16. Splicing efficiencies of the D14 pre-mRNA in the 3-week-old seedlings of WT and dht1.
Supplemental Figure S17. RT-PCR analysis of D14 splicing event in the tiller buds of WT and dht1 mutant.
Supplemental Figure S18. The intron-retained D14 mRNA has a premature stop codon.
Supplemental Figure S19. About 10 μM GR24 treatment slightly induces DHT1 expression in WT seedling.
Supplemental Figure S20. Mutated sites in the D53-CRISPR knockout plants.
Supplemental Figure S21. DHT1 directly binds to the 3′ UTR of D14 mRNA.
Supplemental Figure S22. Gene structures of D14 homologous genes in dicots.
Supplemental Figure S23. Phylogenetic analysis of DHT1 homologous proteins in angiosperms.
Supplemental Figure S24. Mutated sites in knockout plants of SL-related genes.
Supplemental Table S1. Molecular marker primers developed for map-based cloning of the dht1 gene.
Supplemental Table S2. Primers used in this study for vector constructions.
Supplemental Table S3. Primers for RT-qPCR and RT-PCR analyses.
Supplemental Table S4. RNA probe for RNA-EMSA.
Supplemental Table S5. Summary of RNA-seq and RIP-seq reads.
Supplemental Data Set S1. Differential AS events between the WT and dht1 mutant based on RNA-seq (P-value < 0.05).
Supplemental Data Set S2. Analysis of AS events in dht1 mutant according to the position in the gene.
Supplemental Data Set S3. Genes associated with DHT1 binding RNAs (P-value < 0.05).
Supplemental File S1. Text file of the alignment used for the phylogenetic analysis shown in Supplemental Figure S6.
Funding
This work was supported by grants from National Key R&D Program of China (2020YFE0202300) and the National Natural Science Foundation of China (No. 31771765 and No. 92035301).
Conflict of interest statement. The authors declare no conflicts of interest.
Supplementary Material
Contributor Information
Tianzhen Liu, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Xin Zhang, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Huan Zhang, State Key Laboratory for Crop Genetics and Germplasm Enhancement, Jiangsu Plant Gene Engineering Research Center, Nanjing Agricultural University, Nanjing 210095, China.
Zhijun Cheng, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Jun Liu, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Chunlei Zhou, State Key Laboratory for Crop Genetics and Germplasm Enhancement, Jiangsu Plant Gene Engineering Research Center, Nanjing Agricultural University, Nanjing 210095, China.
Sheng Luo, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Weifeng Luo, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Shuai Li, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Xinxin Xing, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Yanqi Chang, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Cuilan Shi, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Yulong Ren, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Shanshan Zhu, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Cailin Lei, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Xiuping Guo, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Jie Wang, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Zhichao Zhao, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Haiyang Wang, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Huqu Zhai, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Qibing Lin, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Jianmin Wan, National Key Facility for Crop Gene Resources and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China; State Key Laboratory for Crop Genetics and Germplasm Enhancement, Jiangsu Plant Gene Engineering Research Center, Nanjing Agricultural University, Nanjing 210095, China.
J.Wan and Q.L. supervised the project. J.Wan, H.W., Q.L., X.Z., H.Zhai, and T.L. designed the research and wrote the paper. Z.C. provided the plant mutant material. T.L. and H.Zhang performed most of the experiments. J.L. and T.L. analyzed the data. H.Zhang and C.Z. performed the subcellular localization assay. S.L., C.S., and W.L. provided the technical assistance. S.L., X.X., and Y.C. performed some of the RT-qPCR analyses and immunoblotting analyses. Y.R., S.Z., and X.G. generated the transgenic plants. C.L., J.Wan, and Z.Z. cultivated the transgenic plants in the field.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) are: Qibing Lin (linqibing@caas.cn) and Jianmin Wan (wanjianmin@caas.cn).
References
- Ahmad A, Dong YZ, Cao XF (2011) Characterization of the PRMT gene family in rice reveals conservation of arginine methylation. PLoS ONE 6: e22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol 66: 161–186 [DOI] [PubMed] [Google Scholar]
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science 335: 1348–1351 [DOI] [PubMed] [Google Scholar]
- Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J (2009) d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol 50: 1416–1424 [DOI] [PubMed] [Google Scholar]
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 [DOI] [PubMed] [Google Scholar]
- Barta A, Kalyna M, Reddy AS (2010) Implementing a rational and consistent nomenclature for serine/arginine-rich protein splicing factors (SR proteins) in plants. Plant Cell 22: 2926–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe BJ (2006) Alternative splicing: new insights from global analyses. Cell 126: 37–47 [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig U, Barbosa-Morais NL, Pan Q, Nachman EN, Alipanahi B, Gonatopoulos-Pournatzis T, Frey B, Irimia M, Blencowe BJ (2014) Widespread intron retention in mammals functionally tunes transcriptomes. Genome Res 24: 1774–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P, Hsieh HY, Tu SL (2021) The U1 snRNP component RBP45d regulates temperature-responsive flowering in Arabidopsis. Plant Cell 34: 834–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HT, Liu HB, Deng Y, Xiao JH, Li XH, Wang SP (2015) The WRKY45-2 WRKY13 WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol 167: 1087–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern M, Bai W, Sze-To WH, Canlas PE, Bartley LE, Ronald PC (2012) A rice transient assay system identifies a novel domain in NRR required for interaction with NH1/OsNPR1 and inhibition of NH1-mediated transcriptional activation. Plant Methods 8: 1746–4811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel G, Kahles A, Kesarwani AK, Stauffer E, Behr J, Drewe P, Ratsch G, Wachter A (2013) Nonsense-mediated decay of alternative precursor mRNA splicing variants is a major determinant of the Arabidopsis steady state transcriptome. Plant Cell 25: 3726–3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J, Bhosale R, Huang G, Pandey BK, Parker H, Zappala S, Yang J, Dievart A, Bureau C, Ljung K, et al. (2018) Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate. Nat Commun 9: 1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohring J, Jacak J, Barta A (2014) Imaging of endogenous messenger RNA splice variants in living cells reveals nuclear retention of transcripts inaccessible to nonsense-mediated decay in Arabidopsis. Plant Cell 26: 754–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Xu Y, Liu H, Mao Z, Zhang C, Ma Y, Zhang Q, Meng Z, Chong K (2013) The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat Commun 4: 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmoko R, Yoo JY, Ko KS, Ramasamy NK, Hwang BY, Lee EJ, Kim HS, Lee KJ, Oh DB, Kim DY, et al. (2016) N-glycan containing a core alpha1,3-fucose residue is required for basipetal auxin transport and gravitropic response in rice (Oryza sativa). New Phytol 212: 108–122 [DOI] [PubMed] [Google Scholar]
- He Y, Smith R (2009) Nuclear functions of heterogeneous nuclear ribonucleoproteins A/B. Cell Mol Life Sci 66: 1239–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6: 271–282 [DOI] [PubMed] [Google Scholar]
- Hoskins AA, Moore MJ (2012) The spliceosome: a flexible, reversible macromolecular machine. Trends Biochem Sci 37: 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Rivers J, Leon P, McQuinn RP, Pogson BJ (2016) Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci 21: 792–803 [DOI] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46: 79–86 [DOI] [PubMed] [Google Scholar]
- Ito Y, Kurata N (2006) Identification and characterization of cytokinin-signalling gene families in rice. Gene 382: 57–65 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Long Y, Zhang H, Li Z, Liu Z, Zhao Y, Lu D, Jin X, Deng X, Xia R, et al. (2020) Post-transcriptional splicing of nascent RNA contributes to widespread intron retention in plants. Nat Plants 6: 780–788 [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, et al. (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445: 652–655 [DOI] [PubMed] [Google Scholar]
- Lambermon MH, Fu Y, Wieczorek Kirk DA, Dupasquier M, Filipowicz W, Lorkovic ZJ (2002) UBA1 and UBA2, two proteins that interact with UBP1, a multifunctional effector of pre-mRNA maturation in plants. Mol Cell Biol 22: 4346–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kinoshita T, Pandey S, Ng CK, Gygi SP, Shimazaki K, Assmann SM (2002) Modulation of an RNA-binding protein by abscisic-acid-activated protein kinase. Nature 418: 793–797 [DOI] [PubMed] [Google Scholar]
- Li M, Xiong GY, Li R, Cui JJ, Tang D, Zhang BC, Pauly M, Cheng ZK, Zhou YH (2009) Rice cellulose synthase-like D4 is essential for normal cell-wall biosynthesis and plant growth. Plant J 60: 1055–1069 [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930 [DOI] [PubMed] [Google Scholar]
- Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, et al. (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21: 1512–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorkovic ZJ, Barta A (2002) Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res 30: 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorkovic ZJ, Wieczorek Kirk DA, Lambermon MH, Filipowicz W (2000) Pre-mRNA splicing in higher plants. Trends Plant Sci 5: 160–167 [DOI] [PubMed] [Google Scholar]
- Mao YS, Zhang B, Spector DL (2011) Biogenesis and function of nuclear bodies. Trends Genet 27: 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris C, Dominguez C, Allain FH (2005) The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J 272: 2118–2131 [DOI] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Guo D, Zhang J, Huang Q, Qin G, Zhang X, Wan J, Gu H, Qu LJ (2013) Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res 23: 1233–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, , WilliamsBA, , McCueK, , SchaefferL, , Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Tran LSP, Van Nguyen D, Fujita M, Maruyama K, Todaka D, Ito Y, Hayashi N, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J 51: 617–630 [DOI] [PubMed] [Google Scholar]
- Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA 91: 5222–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel H, Parra M, Gee SL, Mohandas N, Pachter L, Conboy JG (2016) A dynamic intron retention program enriched in RNA processing genes regulates gene expression during terminal erythropoiesis. Nucleic Acids Res 44: 838–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rino J, Carmo-Fonseca M (2009) The spliceosome: a self-organized macromolecular machine in the nucleus? Trends Cell Biol 19: 375–384 [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, et al. (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416: 701–702 [DOI] [PubMed] [Google Scholar]
- Schmal C, Reimann P, Staiger D (2013) A circadian clock-regulated toggle switch explains AtGRP7 and AtGRP8 oscillations in Arabidopsis thaliana. PLoS Comput Biol 9: e1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O (2015) Unique aspects of plant nonsense-mediated mRNA decay. Trends Plant Sci 20: 767–779 [DOI] [PubMed] [Google Scholar]
- Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, Zhou Q, Xing Y (2014) rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci USA 111: E5593–E5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugay M, Britanova OV, Merzlyak EM, Turchaninova MA, Mamedov IZ, Tuganbaev TR, Bolotin DA, Staroverov DB, Putintseva EV, Plevova K, et al. (2014) Towards error-free profiling of immune repertoires. Nat Methods 11: 653–655 [DOI] [PubMed] [Google Scholar]
- Song X, Lu Z, Yu H, Shao G, Xiong J, Meng X, Jing Y, Liu G, Xiong G, Duan J, et al. (2017) IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Res 27: 1128–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Chou HL, Zhang L, Hwang SK, Starkenburg SR, Doroshenk KA, Kumamaru T, Okita TW (2018) RNA-binding protein RBP-P is required for glutelin and prolamine mRNA localization in rice endosperm cells. Plant Cell 30: 2529–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Luhrmann R (2009) The spliceosome: design principles of a dynamic RNP machine. Cell 136: 701–718 [DOI] [PubMed] [Google Scholar]
- Wang B, Smith SM, Li J (2018) Genetic regulation of shoot architecture. Annu Rev Plant Biol 69: 437–468 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J (2008) Molecular basis of plant architecture. Annu Rev Plant Biol 59: 253–279 [DOI] [PubMed] [Google Scholar]
- Waters MT, Gutjahr C, Bennett T, Nelson DC (2017) Strigolactone signaling and evolution. Annu Rev Plant Biol 68: 291–322 [DOI] [PubMed] [Google Scholar]
- Will CL, Luhrmann R (2011) Spliceosome structure and function. Cold Spring Harb Perspect Biol 3: a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JJ, Au AY, Ritchie W, Rasko JE (2016) Intron retention in mRNA: no longer nonsense: known and putative roles of intron retention in normal and disease biology. Bioessays 38: 41–49 [DOI] [PubMed] [Google Scholar]
- Wu L, Zhang QQ, Zhou HY, Ni FR, Wu XY, Qi YJ (2009) Rice microRNA effector complexes and targets. Plant Cell 21: 3421–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zhu D, Lin X, Miao J, Gu L, Deng X, Yang Q, Sun K, Zhu D, Cao X, et al. (2016) RNA binding proteins RZ-1B and RZ-1C play critical roles in regulating pre-mRNA splicing and gene expression during development in Arabidopsis. Plant Cell 28: 55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Rocha PSCF, Wang ML, Xu ML, Cui YC, Li LY, Zhu YX, Xia XJ (2011) A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234: 47–59 [DOI] [PubMed] [Google Scholar]
- Xu L, Yuan K, Yuan M, Meng X, Chen M, Wu J, Li J, Qi Y (2020) Regulation of rice tillering by RNA-directed DNA methylation at miniature inverted-repeat transposable elements. Mol Plant 13: 851–863 [DOI] [PubMed] [Google Scholar]
- Xu MR, Huang LY, Zhang F, Zhu LH, Zhou YL, Li ZK (2013) Genome-Wide phylogenetic analysis of stress-activated protein kinase genes in rice (OsSAPKs) and expression profiling in response to Xanthomonas oryzae pv. oryzicola infection. Plant Mol Biol Rep 31: 877–885 [Google Scholar]
- Yang C, Ma B, He SJ, Xiong Q, Duan KX, Yin CC, Chen H, Lu X, Chen SY, Zhang JS (2015) MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 regulate ethylene response of roots and coleoptiles and negatively affect salt tolerance in rice. Plant Physiol 169: 148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, Fu JX, Li J, Cheng XL, Li F, Dong JF, Liu ZL, Zhuang CX (2014a) A novel co-immunoprecipitation protocol based on protoplast transient gene expression for studying protein-protein interactions in rice. Plant Mol Biol Rep 32: 153–161 [Google Scholar]
- Yang Y, Crofts AJ, Crofts N, Okita TW (2014b) Multiple RNA binding protein complexes interact with the rice prolamine RNA cis-localization zipcode sequences. Plant Physiol 164: 1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Ming Z, Yan L, Li S, Wang F, Ma S, Yu C, Yang M, Chen L, Chen L, et al. (2016) DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 536: 469–473 [DOI] [PubMed] [Google Scholar]
- Yap K, Lim ZQ, Khandelia P, Friedman B, Makeyev EV (2012) Coordinated regulation of neuronal mRNA steady-state levels through developmentally controlled intron retention. Genes Dev 26: 1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap WC, Namasivayam P, Ho CL (2014) HnRNP-like proteins as post-transcriptional regulators. Plant Sci 227: 90–100 [DOI] [PubMed] [Google Scholar]
- Zhang SZ, Wu T, Liu SJ, Liu X, Jiang L, Wan JM (2016) Disruption of OsARF19 is critical for floral organ development and plant architecture in rice (Oryza sativa L.). Plant Mol Biol Rep 34: 748–760 [Google Scholar]
- Zhang Y, Gu L, Hou Y, Wang L, Deng X, Hang R, Chen D, Zhang X, Zhang Y, Liu C, et al. (2015) Integrative genome-wide analysis reveals HLP1, a novel RNA-binding protein, regulates plant flowering by targeting alternative polyadenylation. Cell Res 25: 864–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, et al. (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LH, Zhou XE, Yi W, Wu Z, Liu Y, Kang Y, Hou L, de Waal PW, Li S, Jiang Y, et al. (2015) Destabilization of strigolactone receptor DWARF14 by binding of ligand and E3-ligase signaling effector DWARF3. Cell Res 25: 1219–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, et al. (2013) D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504: 406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Liu CF, Liu AL, Zou D, Chen XB (2012) Overexpression of OsHsp17.0 and OsHsp23.7 enhances drought and salt tolerance in rice. J Plant Physiol 169: 628–635 [DOI] [PubMed] [Google Scholar]
- Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L (2006) The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J 48: 687–698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.