Abstract
Allograft rejection is one of the obstacles in achieving a successful vascularized composite allotransplantation (VCA). Treatments of graft rejection with lifelong immunosuppression (IS) subject the recipients to a lifelong risk of cancer development and opportunistic infections. Cell therapy has recently emerged as a promising strategy to modulate the immune system, minimize immunosuppressant drug dosages, and induce allograft tolerance. In this review, the recent works regarding the use of cell therapy to improve allograft outcomes are discussed. The current data supports the safety of cell therapy. The suitable type of cell therapy in allotransplantation is clinically dependent. Bone marrow cell therapy is more suitable for the induction phase, while other cell therapies are more feasible in either the induction or maintenance phase, or for salvage of allograft rejection. Immune cell therapy focuses on modulating the immune response, whereas stem cells may have an additional role in promoting structural regenerations, such as nerve regeneration. Source, frequency, dosage, and route of cell therapy delivery are also dependent on the specific need in the clinical setting.
Keywords: Vascularized composite allotransplantation, Clinical transplantation, Cell therapy, Stem cell, Immune cell
Vascularized composite allotransplantation (VCA) has become a reliable treatment alternative for restoring form and function in patients with significant tissue loss. Various types of clinical VCA, including partial-face, full-face, hand, and knee allotransplantation, have been successfully performed with excellent short-to intermediate-term functional and immunological outcomes [[1], [2], [3], [4]]. Unlike solid organ transplantation (SOT), VCAs usually contain multiple tissue types, including skin, muscle, bone, vessel and nerve. Therefore, the immunogenicity of different tissues in a single composite allograft must be considered. Lifelong IS regimens are required to maintain allograft survival. However, undesired side effects, such as opportunistic infection, metabolic complications, and malignant diseases occur frequently [5].
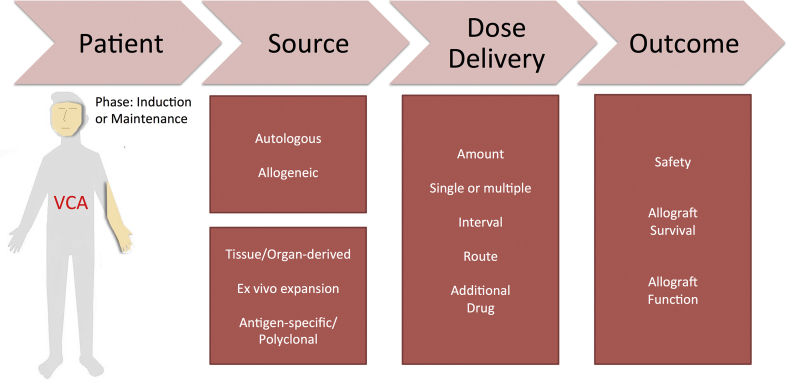
Alternative treatments to overcome immune rejection and to improve allograft outcome have been investigated in the past two decades. Cell therapy is one of the potential strategies to modulate immune systems and allow the recipients to develop tolerance to the allograft. In this review, the recent progress and efforts to induce and maintain allograft acceptance across the full major histocompatibility (MHC) barrier through cell therapy are summarized. The discussion parameters include timing, frequency, and infusion doses of various cell therapies; the comparisons of the autologous and allogenic source of cellular lineage used in cell therapy; the delivery dose and route of cell therapy and the need of the additional adjuvant IS and finally the mechanisms and outcome of using cell therapy in VCAs [Fig. 1].
Fig. 1.
Roadmap of technical considerations regarding the use of cell therapy.
Types of cells for therapy
Cell therapy is defined as the therapeutic use of cellular material through injection, grafting, or implantation into the recipient. The concept of cell therapy was first described in the 19th century with xenocelullar injection to human subjects [6]. Human cells were later developed for therapeutic purpose. Graft rejection is an immune cell-mediated phenomenon; therefore, immune and stem cell-based therapeutic approaches were highly regarded with great potential. These cells may modulate the immune system and induce graft tolerance, proposedly through reducing the graft destructive T cells or the allograft-reacting immune cells. In addition, the anti-inflammatory properties of stem cells via their secretion of anti-inflammatory molecules, such as interleukin(IL)-10, may also dampen the process of immune-mediated graft rejection.
Immune cell therapy
In the investigation of immune cell therapy, the use of dendritic cells (DCs) was the first to be explored. As an antigen-presenting cell, DCs are critical for initiating the immune response. Regulatory DCs (DCregs) play an essential role in maintaining tolerance. They have been applied as cell therapy since 1996. Similar to general DCs, DCregs uptake, process, and present soluble antigen (Ag) [7]. Meanwhile, regulatory T cells (Tregs) are also widely investigated as potential cell therapy in VCA. Tregs are known as a subpopulation of T cells with a function to suppress effector immune cells through activation-induced cell death, “ignorance” of self-antigens [8], and the induction of cell anergy. The later discoveries of CD8 Tregs and Type 1 regulatory T cells were identified as subgroups of Tregs [9,10]. Other regulatory cells with anti-inflammatory properties are regulatory B cells (Bregs) and regulatory macrophages (Mregs). However, to date, only limited VCA studies were performed to investigate these regulatory cells. The potential Bregs as cell therapy to induce tolerance was demonstrated in an islet model [11].
Stem cell therapy
Regarding their ability to differentiate, stem cells can be classified into totipotent, pluripotent, multipotent, oligopotent and unipotent, and all have self-renewal ability. Transferring the donor bone marrow (BM) cells that contain hematopoietic stem cells (HSCs) to the recipient is proven to induce tolerance through chimerism in the animal models [12,13] and clinical VCA studies [14]. Pluripotent HSCs have been used in transplantation since the 1960s and could improve allograft outcomes in SOT [15]. In VCA, these stem cells were studied extensively in small and large animals [[16], [17], [18]]. Other stem cells therapies used in VCA include BM-derived mesenchymal stem cells (BM-MSCs), and adipocytes derived mesenchymal stem cells (AD-MSCs). In addition to the immunological benefit in VCA, they also enhance the process of nerve regeneration [19,20].
Timing and frequency of cell therapy
In general, there are three critical phases of graft acceptance in transplantation: tolerance induction, tolerance maintenance, and rejection treatment. Timing and frequency of cell therapy administration significantly affect allograft and recipient outcomes [21]. In animal studies, the cells are mostly infused on the same day of transplantation or given multiple doses within the first week post-transplantation. The induction phase is the most critical period for recipient immune system activation. Those infused cells are aimed to modulate the immune reaction, allowing the reduction of immunosuppressants dosage. Among the potential cellular candidates, BM cells [13] and DCregs [22] were studied comprehensively in immune tolerance induction. Several publications also reported the use of cell therapy in the maintenance phase and allograft rejection. In particular, Tregs [23] and umbilical cord derived mesenchymal stem cells [24] have been used to rescue rejections. The frequency of injections also affects the outcome of transplantation. Repetitive cell injections have cumulative beneficial effects. The extracellular vesicles and graft outcome are positively correlated with the number of cell injections [25,26]. Although several studies have demonstrated the effectiveness of a single bolus cell injection in graft tolerance induction [Table 1], the same quantity of AD-MSCs in the divided doses given repeatedly may lead to a superior VCA outcome [27]. However, conflicting results were reported [28,29]. A similar multiple injection approach with DCs was used in SOT clinical trials, which resulted in potentially conducive to modulate anti-donor immune reactivity [30].
Table 1.
Immune cell therapy in the VCA animal study.
| Literature (Year) | Cell Type | Species | Model | Dosage (Day of administration), Route | Induction and IS (Day of administration) | Outcome | Mechanism |
|---|---|---|---|---|---|---|---|
| Kuo et al. (2009) [22] | Alloantigen-stimulated DCs | Rat | Hindlimb | 0.7-1x107 (7,14,21), IV |
ALS (−4,1), CsA (0–20) | Prolong allograft >200 days | Induce T cell donor-specific hyporesponsiveness |
| Cheng et al. (2018) [39] | Alloantigen-stimulated CD4+CD25+Tregs |
Rat | Hindlimb | 7 × 105-2x106 (10), IV | ALS (−1,2) + CsA (0–7) | Maintenance of graft acceptance | Tregs graft engraftment |
| Anggelia et al. (2021) [23] | CD4+CD25+Tregs from tolerant recipients | Mouse | Osteo-myocutaneous | 2 × 106 (30), IV |
CoB (0,2) + RPM (0–28) | Rescue graft rejection | Suppress donor specific T cell proliferation |
| Lin et al. (2013) [67] | CD4−CD8-Tregs | Mouse | Osteo-myocutaneous | 5 × 106 (0), IV |
ALS | Prolong allograft >180 days | Induce T cell donor-specific hyporesponsiveness |
| Radu et al. (2012) [42] | Donor Mregs | Rat | Osteo-myocutaneous | 5 × 106 (0), IV and IM |
NA | Extended allograft survival from 5.6 to 7.4 days | Reduce infiltration of inflammatory cells |
Abbreviations: ALS: anti-lymphocytes serum; CsA: cyclosporine A; CoB: costimulation blockade; DCs: dendritic cells; IM: intramuscular; IS: immunosuppression; IV: intravenous; Mregs: regulatory macrophages; Tregs: regulatory T cells; NA: not applicable; RPM: rapamycin.
Source and expansion
The manufacturing of the therapeutic cells should adhere strictly to Good Manufacturing Practices. The source of the cell can be derived either from allogeneic or autologous cellular lineages. Autologous cells are obtained from the recipient patient and cultured in smaller numbers, whereas allogeneic cells are obtained from unrelated donor tissues (such as BM) and manufactured in large quantities. In allogeneic cell therapies, cell manufacturing is aimed to treat on the community level with comprehensive product quality control. In contrast, the autologous cells are custom-made, and a small quantity is manufactured in hospital-affiliated laboratories. Autologous cell transfer might be a safer strategy than allogenic cell infusion for transplant recipients. It minimizes the risk of alloantigen-mediated attack on the infused cells generated from the recipient, thus providing better therapeutic efficacy. Studies using autologous cell infusions demonstrated a modest to significant beneficial effect in rat and swine VCA studies [27,31]. Clinically, the use of autologous cells was tried successfully in double hand transplantation [32]. Due to the limitation of the autologous cell number, the ex vivo cell expansion is required. However, allogenic cells could be used as the alternative to autologous cell therapy to increase the source of cell transplantation [17,18]. The tissue origins of cells also influence their immunomodulatory effectiveness, for example, AD-MSCs were shown to have greater immunomodulatory properties than BM-MSCs [33].
Besides the source, other factors could also affect the efficacy. In canines, age is an influential factor of BM-MSCs proliferation and immunomodulatory properties. With advanced age, the osteogenic gene expression is increased under proinflammatory conditions. It was suggested the BM-MSCs from young donors could be used as an alternative source to autologous BM-MSCs therapy for older canines [34]. The level of antigen specificity can also affect the suppressive capabilities of cell therapy. The treatment with antigen-specific Tregs is more effective than the treatment with non-specific Tregs [35]. A good example is that the activation of natural Tregs via the A2-CAR induced proliferation enhances the suppressor effect of the modified natural Tregs [36]. Modified microparticles to sustainably release IL-2, transforming growth factor(TGF)-β, and rapamycin also successfully induced Tregs differentiation from naïve T cells, demonstrated enhancement of Tregs associated cytokines expression and finally prolonged survival time of rodent VCA allograft [37,38].
Route of administration and additional immunosuppression (IS)
There are 2 main routes of cell therapy administration in VCA. One is via the circulation system, and the other is through the local injection. Intravenous (IV) administration of the therapeutic cells is more commonly used in transplantation due to the migratory nature of the immune and stem cells into the allograft, allowing adequate delivery of the cells to the targeted site. However, through IV injection, the cells can migrate to non-targeted organs such as the spleen, lymph nodes [39] or trapped in undesirable sites such as the lung [40,41]. The systemic delivery of the cells appears to be well tolerated; no significant adverse effects were reported despite the infusion of large cell quantity.
The local intramuscular injection has been used as an alternative route of infusion, and no significant difference was demonstrated in allograft outcome [42]. Considering the efficacy and efficiency of cell administration, the local administration might reduce the required therapeutic cellular number in cell therapy. A comprehensive study is needed to verify this hypothesis in VCA.
Cell therapy alone in the early stage of transplantation is not sufficient to induce long-term graft acceptance. Without immunosuppressants, Tregs infusion only prolonged skin allograft survival for 25 days. When T cell depletion induction agents and maintenance immunosuppressants were used in conjunction with Tregs infusion, heart allograft can survive beyond 125 days [43]. Thus, it is still mandatory to use induction agents (polyclonal or monoclonal antibodies) to deplete thymocytes, T- and B-cells in the induction phase despite the use of Tregs infusion. Interfering agents [anti-lymphocyte serum (ALS) or costimulation blockade in animal studies and thymoglobulin or alemtuzumab in clinical trials] can be used in the induction phase to interrupt the adaptive immune response against alloantigen in conjunction with Tregs infusion to achieve allograft tolerance [14,23,27,39,44]. Strategies to expand Tregs with a low dose of IL-2 administration after Tregs infusion [45,46].
Mechanism and outcome
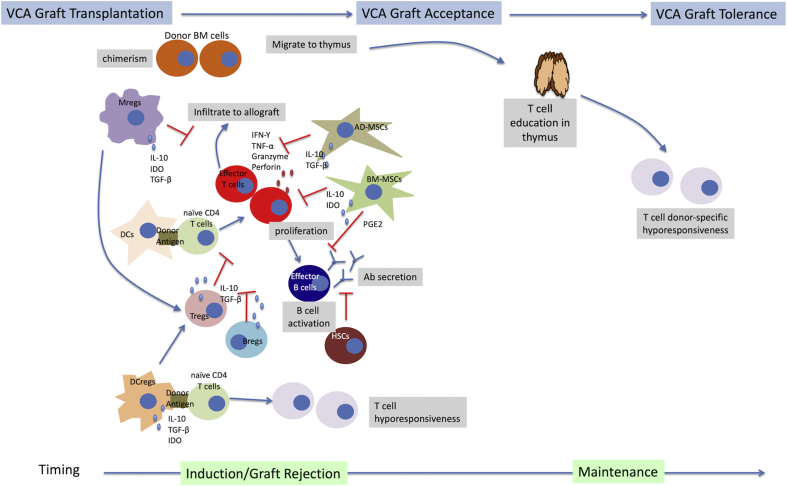
The expected outcome with cell therapy in VCA is achieving donor-specific tolerance by targeting peripheral and central mechanisms with minimize the use of immunosuppressants. Donor-specific tolerance could be achieved through chimerism that is defined as a single organism that contains cells derived from more than one origin. Several mechanisms of chimerism-induced peripheral and central tolerance have been proposed [47]. Many experimental studies from murine to large animals and clinical studies have suggested chimerism plays an active role in the induction and maintenance phase of allograft tolerance. Mixed chimerism could be achieved by conventional BM transplantation or vascularized bone marrow (VBM) transplantation. BM produces factors including chemokines, cytokines, adhesion molecules, and extracellular matrix proteins. The niche provided by BM is crucial for HSCs expansion and repopulation. Moreover, VBM transplantation tends to induce less graft-versus-host disease than conventional BM transplantation. Our recent study and other previous studies directly proved the superior efficacy of VBM transplantation to conventional BM transplantation [13,48,49]. However, its application in a clinical setting is far from realized. A clinical study by Schneeberger et al. showed the infusion of donor BM was ineffective to induce chimerism in patients who received VCA [14]. Donor BM cells migrate from the donor femur to recipients’ organ, such as thymus [13,23,50], a place where T cell education occurs [51]. Chimerism in thymus indicates the central tolerance is developed, since the donor reactive Vβ5-expressing T cells in the Balb/c to B6 murine models were specifically suppressed in tolerant recipients [13,23,50]. The crucial roles of thymus in induction of donor-specific tolerance have been also reported, where thymectomy affected generation of donor specific chimerism in VCA recipients [[52], [53], [54]].
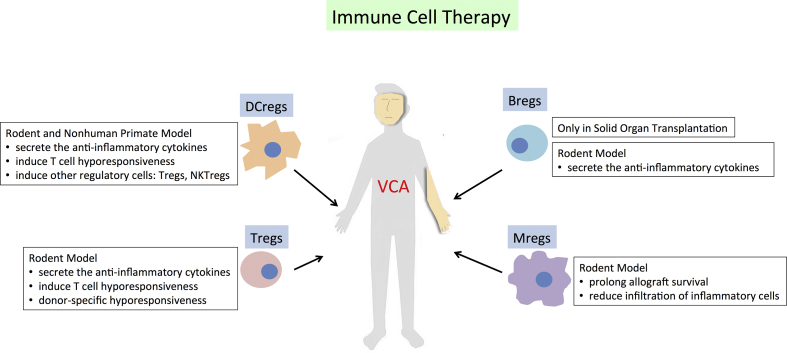
On the other hand, the immune cells that possess anti-inflammatory properties promote allograft acceptance through effector cells suppression [Fig. 2, Fig. 3, Fig. 4]. DCregs have been demonstrated in rat hindlimb models to promote transplantation tolerance through mitigation of T cell activation and induction of T cell anergy, T cell deletion, and Tregs conversion [55]. The evidence of Tregs involved in transplantation tolerance also has been widely reported. In SOT, increasing circulatory and graft-infiltrating Tregs are mainly related to donor-specific hyporesponsiveness [56,57]. The rodent VCA studies also support the importance of Tregs to promote allograft tolerance. CD4+CD25+ Tregs from the tolerant recipients were shown to have a higher suppressive capacity to effector T cells than those from the rejected. CD4+CD25+ Tregs from the tolerant recipients also migrated to the allograft to maintain the graft tolerance [39,58]. Tregs migration is associated with chemokine [59]. CCR7 involved in Tregs homing to secondary lymphoid organ while CCR4 responsible for Tregs migration to skin [60]. Learning from phenomenon in tumor microenvironment where tumor cells secrete CCL22 to recruit Tregs through interaction with CCR4 expressed on Tregs [61,62], strategy using synthetic of this chemokine to induce preferential Tregs migration to allograft and prolong the survival indefinitely might be a potential for clinical translation [63]. Although several conflicting results were reported in nonhuman primate studies [64] where Tregs infusion could increase memory T cell and alloantibody responses [65], the majority of evidences showed the advantage of Tregs infusion to prevent rejection. In a bilateral hand VCA recipient, the number of Foxp3+ cells increased in allograft skin over 6 years post-transplantation [66]. The utilization of non-CD4+ Tregs (CD4−CD8- Tregs) in osteomyocutaneous allotransplantation showed infusion induced donor-specific tolerance to allografts in mice that concurrently received ALS and short-term immunosuppressants [67]. However, the reliable and reproducible method for the isolation and expansion of non-CD4+ Tregs are not established yet.
Fig. 2.
The proven immunomodulatory mechanisms of Immune cell therapy in the animal VCA study.
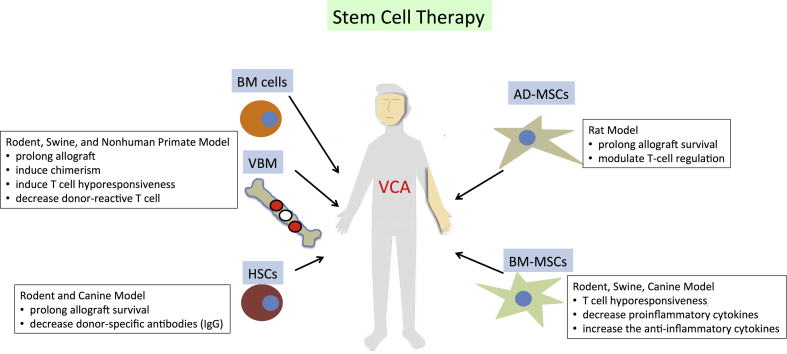
Fig. 3.
The proven immunomodulatory mechanisms of stem cell therapy in the animal VCA study.
Fig. 4.
Illustration of immune cell and stem cell immunomodulatory mechanisms in VCA transplantation.
The potential use of stem cell therapy has also been demonstrated [Table 2 and Fig. 3, Fig. 4]. Cellular and molecular studies showed their anti-inflammatory [68] and immunomodulatory activities [69]. In VCA, syngeneic HSC transplantation with conventional immunosuppressants effectively decreased donor-specific antibodies (IgG) and increased allograft survival in the sensitized rats [16]. In the canine model, simultaneous HSC transplantation and VCA also induced allograft tolerance [17], and the additional use of granulocyte colony-stimulating factor can enhance HSC engraftment [18]. BM-MSCs are multipotent stem/progenitor cells. They are essential for the development of stable mixed chimerism, which is vital for donor-specific tolerance induction in the recipient. It has been hypothesized that BM-MSCs may prolong VCA survival through the increased levels of CD4+CD25+ Tregs, TGF-β1, and serum IL-10 [70]. A recent study illustrated the induced pluripotent stem cell-derived mesenchymal stem cells (MSCs) also prolonged VCA allograft survival through the induction of T cell hyporesponsiveness, reduction of proinflammatory cytokines (interferon gamma and tumor necrosis factor α), and elevation of the anti-inflammatory cytokine, IL-10 [71]. AD-MSCs also prolonged VCA allograft survival by suppressing effector T cells and enhancing Tregs [Table 2] [72,73]. Not only preventing immune rejection, BM-MSCs and AD-MSCs were also reported to enhance nerve regeneration through the secretion of neuroregulatory factors and the ability to differentiate into Schwann-like cells [74].
Table 2.
Stem cell therapy in the VCA animal study.
| Literature (Year) | Cell Type | Species | Model | Dosage (Day of administration), Route | Induction and IS (Day of administration) | Outcome | Mechanism |
|---|---|---|---|---|---|---|---|
| Xu et al. (2013) [12] | Donor BM cells |
Rat | Osteo-myocutaneous | 1.5 × 108 (0), IV | Anti-TCR (−3) + TBI (−1) + ALS (9) +Tac (−1–9) | Prolong allografts >200 days | Thymic deletion alloreactive T cell, superior donor-specific Tregs |
| Lin et al. (2021) [13] | Donor BM cells |
Mouse | Myocutaneous | 1.5 × 108 (0), IV | CoB (0,2) + RPM (0–28) | Prolong allografts >120 days | Induce chimerism and donor-specific tolerance |
| Mathes et al. (2014) [17] | Donor HSCs |
Canine | Hindlimb | 1.7-5.1 × 108/kg (0), IV | TBI (−1) + CsA (-1-35) + MMF (0–28) | Prolong allografts >62 weeks | Increase Tregs engraftment, induce donor-specific tolerance |
| Leonard et al. (2014) [81] | Donor HSCs |
Swine | Fascio-cutaneous | 15 × 109 (0), IV |
T cell depletion + non-myeloablatıve TBI + CsA (0–45) |
Prolong allograft 85–100 days | Induce peripheral blood chimerism between 20 and 100% |
| Mitsuzawa et al. (2019) [71] | Syngeneic iMSCs | Rat | Hindlimb | 2 × 106 (7), IV | Tac (0–6) | Prolong allografts >17–21 days | Induce T cell donor-specific hyporesponsiveness |
| Kuo et al. (2017) [31] | Autologous AD-MSCs |
Swine | Hindlimb | 1 × 106/kg (0–3), IV | TBI (−1) + Tac (0–4) |
Prolong allografts >196 days | Modulate T cell regulation |
| Plock et al. (2017) [27] | Syngeneic AD-MSCs |
Rat | Hindlimb | 1 × 106 (4,8,15) IV |
ALS (−4,1), Tac (0–21) | Prolong 50% allografts >100 days | Induce chimerism and donor-specific tolerance, elevate Tregs |
| Kuo et al. (2011) [73] | Donor AD-MSCs |
Rat | Hindlimb | 2 × 106 (7,14,21), IV | ALS (−4,1) + CsA (0–20) | Prolong allografts >200 days | Suppress T cell and enhance Tregs proliferation |
| Cheng et al. (2014) [29] | Syngeneic AD-MSCs |
Rat | Osteo-myocutaneous | 2 × 106 (1), IV | TBI (−1) + ALG (−1,10) + CsA (0–10) | Prolong 67% allografts >140 days | Induce donor-specific tolerance, elevated circulating Tregs |
| Schweizer et al. (2020) [82] | Syngeneic AD-MSCs |
Rat | Hindlimb | 1 × 106 (2,4,7,14,28), IV |
CTLA4Ig (2,4,7) + ALS (1,5) + Tac (0–14) | Prolong 86% allografts >120 days | Induce chimerism and elevated systemic and skin Tregs |
Abbreviations: AD-MSCs: adipose derived mesenchymal stem cells; ALG: anti-lymphocyte globulin; ALS: anti-lymphocytes serum; BM: bone marrow; CsA: cyclosporine A; CoB: costimulation blockade; DCs: dendritic cells; IM: intramuscular; iMSCs: induced mesenchymal stem cells; IS: immunosuppression; IV: intravenous; NA: not applicable; RPM: rapamycin; Tac: tacrolimus; TBI: total body irradiation.
Clinical trials: learning from solid organ transplantation (SOT)
Studies of SOT have been broadly performed since decades ago. The results provided significant insights into the mechanisms of rejection and tolerance. These experiences help delineate the immune responses to the allografts, and in the long-term, design optimal IS regimens in conjunction with cell-based therapies. DCregs, Tregs, and BM-MSCs are currently used in transplantation phase I/II clinical trials [30,75,76]. Although all these cells play the regulatory roles to suppress immune responses, only CD4+ regulatory cells or Tregs consistently exert active tolerizing functions by inhibiting immune effector mechanisms [77]. Therefore, new IS protocols are developed to facilitate Tregs induction and expansion after transplantation [78]. The long-term results of these studies are highly anticipated with the hope to improve the lives of transplant recipients fundamentally. In recently studies from the phase I/II clinical trials in SOT, cell therapy showed good efficacy without adverse effects from short to mid-term monitoring [Table 3] [24,78,79]. Listed minor side effects include creatinine elevation, cytomegalovirus viraemia, urinary tract infections that can be treated promptly. Nevertheless, the application of cell therapy in VCA remains limited [80]; this may be primarily due to small case number of VCA. But some studies have shown promising results. The Pittsburgh protocol demonstrated the use of BM cell therapy in VCA could induce chimerism. Thus, the dosage of IS monotherapy can be tapered [14]. The pilot study of MSCs therapy in bilateral hands also showed favorable results [32].
Table 3.
Cell therapy in clinical transplantation.
| Literature (Year) | Cell Type | Model | Phase | Dosage (Days of administration), Route | Induction and IS (Days of administration) | Outcome |
|---|---|---|---|---|---|---|
| Schneeberger et al. (2013) [14] | Donor BM cells | Upper Extremity | I | 5–10 × 108/kg (14), IV | Alemtuzumab (0) + methylprednisolone (0) + tapered Tac | Safe and allows to use low-dose Tac monotherapy Adverse event: increase serum creatinine, hyperglycemia, hyperuricemia, minor wound infections |
| Del Bene et al. (2013) [32] | Autologous MSCs | Bilateral Hand Transplant | I | 2 × 106 (1), IV | Basiliximab (4) + methylprednisolone (0–6) cortisone + Tac + MMF | Graft acceptance up to 10 months |
| Macedo et al. (2020) [30] | Donor-derived DCregs | Liver | I | 2.5-10 × 106 (−7, −3, 0), IV |
Mycophenolic acid | Safe and induce systemic changes in recipient antigen presenting cells and T cells Adverse event: NA |
| Todo et al. (2016) [83] | Donor-specific Tregs | Liver | I | 0.23–6.37 × 106/kg (13), IV | Steroids + MMF (0–30) + Tac/CsA/RPM + CP (5) | Normal function 3/10 Patients show mild rejection after 1 year transplant Adverse event: CMV antigenemia, diabetic nephropathy |
| Sawitzki et al. (2020) [84] | CBMPs (DCregs, Mregs, Tregs) | Kidney | I/IIa | 2.0–2.5 × 106/kg (−1 or 7), IV | Basiliximab + tapered steroids, MMF, and Tac | Weaned IS Adverse event: Increase creatinine |
| Sánchez-Fueyo et al. (2020) [44] | AutologousPolyclonal Tregs | Liver | I | 1-4.5 × 106 (90), IV |
Thymoglobulin (1–7) + Tac + methylprednisolone (700) | Reduced anti-donor T cell responses Adverse event: NA |
| Mathew et al. (2018) [78] | Polyclonal Tregs | Kidney | I | 0.5, 1, and 5 × 109 (60), IV | Alemtuzumab (0,2)+ Tac (-1-30) + Sirolimus (replace Tac) + Mycophenolate | No rejection up to 2 years Adverse event: NA |
| Roemhild et al. (2020) [85] | Polyclonal Tregs | Kidney | I/IIa | 0.5, 1.0, or 2.5-3.0 × 106/kg (7), IV |
Prednisone (0–98) + MMF (0–336) + Tac |
Enable minimization of IS Adverse event: CMV viraemia, urinary tract infection, and pneumonia |
| Hutchinson et al. (2011) [41] | Donor-derived Mregs |
Kidney | I | 5 × 107 (−6/-7) IV |
Steroid (0–700) + Tac | Stable kidney function up to 3 years with minimal maintenance IS Adverse event: NA |
| Detry et al. (2017) [76] | Donor MSCs | Liver | I | 3 × 106/kg (3), IV |
ATG + Tac (0–270) + MMF (0–365) + Steroid | Did not promote tolerance Adverse event: NA |
| Shi et al. (2017) [24] | UC-MSCs | Liver | I | 3 × 106/kg during acute rejection, IV |
Basiliximab + tapered corticosteroids + prednisolone (0–90) + MMF + Tac | UC-MSC infusion for acute graft rejection is feasible Adverse event: NA up to 24 weeks |
Abbreviations: ATG: anti-thymocytes globulin; CsA: cyclosporine A; CBMPs: cell-based medicinal products; CMV: cytomegalovirus; CP: cyclophosphamide; DCregs: regulatory dendritic cells; IS: immunosuppression; IV: intravenous; MMF: mycophenolate mofetil; Mregs: regulatory macrophages; MSCs: mesenchymal stem cells; NA: not applicable; RPM: rapamycin; Tac: tacrolimus; Tregs: regulatory T cells; UC-MSCs: umbilical cord mesenchymal stem cells.
Summary
Conventional strategies to prevent allograft rejection in VCA rely on long-term IS. Recent data demonstrated the potential application of cell therapy in VCA. Animal studies elucidated the plausibility of using both immune cells and stem cells as the adjuvant treatment to improve VCA outcome and minimize the use of long-term immunosuppressants. Thus, side effects of long-term IS can be reduced. The selection of suitable cell therapy is clinically dependent. BM cells are more feasible for chimerism induction in the early phase after transplantation. Immune cell therapy focuses on modulating the alloreactive response. In addition, tissue-derived MSCs could be used to facilitate nerve regeneration. The source, frequency, dosage, and route of cell therapies depend on the clinical need. Although evidences from clinical VCA trials are still limited, the use of autologous cell therapy in clinical SOT trials with either bolus or divided doses is considered to be safe with good efficacy.
Funding
This work was financially supported by the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-182A-027-MY3 and 110-2811-B-182A-513) and Chang Gung Medical Foundation, Taiwan (CPRPG3F0035).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This work was financially supported by the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-182A-027-MY3 and 110-2811-B-182A-513) and Chang Gung Medical Foundation (CPRPG3F0035).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Broyles J.M., Alrakan M., Ensor C.R., Khalifian S., Kotton C.N., Avery R.K., et al. Characterization, prophylaxis, and treatment of infectious complications in craniomaxillofacial and upper extremity allotransplantation: a multicenter perspective. Plast Reconstr Surg. 2014;133:543e–551e. doi: 10.1097/PRS.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 2.Coffman K.L., Siemionow M.Z. Ethics of facial transplantation revisited. Curr Opin Organ Transplant. 2014;19:181–187. doi: 10.1097/MOT.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 3.Pomahac B., Gobble R.M., Schneeberger S. Facial and hand allotransplantation. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siemionow M.Z., Zor F., Gordon C.R. Face, upper extremity, and concomitant transplantation: potential concerns and challenges ahead. Plast Reconstr Surg. 2010;126:308–315. doi: 10.1097/PRS.0b013e3181dcb6f4. [DOI] [PubMed] [Google Scholar]
- 5.Petruzzo P., Dubernard J.M. The international registry on hand and composite tissue allotransplantation. Clin Transpl. 2011:247–253. [PubMed] [Google Scholar]
- 6.Lefrere J.J., Berche P. Doctor Brown-Sequard's therapy. Ann Endocrinol. 2010;71:69–75. doi: 10.1016/j.ando.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Ezzelarab M., Thomson A.W. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23:252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Q., Bluestone J.A., Kang S.M. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieyra-Lobato M.R., Vela-Ojeda J., Montiel-Cervantes L., Lopez-Santiago R., Moreno-Lafont M.C. Description of CD8(+) regulatory T lymphocytes and their specific intervention in graft-versus-host and infectious diseases, autoimmunity, and cancer. J Immunol Res. 2018;2018:3758713. doi: 10.1155/2018/3758713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pot C., Apetoh L., Kuchroo V.K. Type 1 regulatory T cells (Tr1) in autoimmunity. Semin Immunol. 2011;23:202–208. doi: 10.1016/j.smim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuetz C., Lee K.M., Scott R., Kojima L., Washburn L., Liu L., et al. Regulatory B cell-dependent islet transplant tolerance is also natural killer cell dependent. Am J Transplant. 2017;17:1656–1662. doi: 10.1111/ajt.14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H., Ramsey D.M., Wu S., Bozulic L.D., Ildstad S.T. Simultaneous bone marrow and composite tissue transplantation in rats treated with nonmyeloablative conditioning promotes tolerance. Transplantation. 2013;95:301–308. doi: 10.1097/TP.0b013e31827899fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C.H., Anggelia M.R., Cheng H.Y., Wang A.Y.L., Chuang W.Y., Lin C.H., et al. The intragraft vascularized bone marrow component plays a critical role in tolerance induction after reconstructive transplantation. Cell Mol Immunol. 2021;18:363–373. doi: 10.1038/s41423-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneeberger S., Gorantla V.S., Brandacher G., Zeevi A., Demetris A.J., Lunz J.G., et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg. 2013;257:345–351. doi: 10.1097/SLA.0b013e31826d90bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenecke C., Hertenstein B., Schetelig J., van Biezen A., Dammann E., Gratwohl A., et al. Solid organ transplantation after allogeneic hematopoietic stem cell transplantation: a retrospective, multicenter study of the EBMT. Am J Transplant. 2010;10:1897–1906. doi: 10.1111/j.1600-6143.2010.03187.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang H.D., Fidder S.A.J., Miller D.T., Furtmuller G.J., Ahmadi A.R., Nagele F., et al. Desensitization and prevention of antibody-mediated rejection in vascularized composite allotransplantation by syngeneic hematopoietic stem cell transplantation. Transplantation. 2018;102:593–600. doi: 10.1097/TP.0000000000002070. [DOI] [PubMed] [Google Scholar]
- 17.Mathes D.W., Chang J., Hwang B., Graves S.S., Storer B.E., Butts-Miwongtum T., et al. Simultaneous transplantation of hematopoietic stem cells and a vascularized composite allograft leads to tolerance. Transplantation. 2014;98:131–138. doi: 10.1097/TP.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang J., Graves S.S., Butts-Miwongtum T., Sale G.E., Storb R., Mathes D.W. Long-term tolerance toward haploidentical vascularized composite allograft transplantation in a canine model using bone marrow or mobilized stem cells. Transplantation. 2016;100:e120–e127. doi: 10.1097/TP.0000000000001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez D.N.R., Bertanha M., Fernandes T.D., Resende L.A.L., Deffune E., Amorim R.M. Effects of canine and murine mesenchymal stromal cell transplantation on peripheral nerve regeneration. Int J Stem Cells. 2017;10:83–92. doi: 10.15283/ijsc16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masgutov R., Masgutova G., Mukhametova L., Garanina E., Arkhipova S.S., Zakirova E., et al. Allogenic adipose derived stem cells transplantation improved sciatic nerve regeneration in rats: autologous nerve graft model. Front Pharmacol. 2018;9:86. doi: 10.3389/fphar.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Q., Huang Z., Han F., Zhao M., Cao R., Zhao D., et al. Allogeneic mesenchymal stem cells as induction therapy are safe and feasible in renal allografts: pilot results of a multicenter randomized controlled trial. J Transl Med. 2018;16:52. doi: 10.1186/s12967-018-1422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo Y.R., Huang C.W., Goto S., Wang C.T., Hsu L.W., Lin Y.C., et al. Alloantigen-pulsed host dendritic cells induce T-cell regulation and prolong allograft survival in a rat model of hindlimb allotransplantation. J Surg Res. 2009;153:317–325. doi: 10.1016/j.jss.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Anggelia M.R., Cheng H.Y., Chuang W.Y., Hsieh Y.H., Wang A.Y.L., Lin C.H., et al. Unraveling the crucial roles of FoxP3+ regulatory T cells in vascularized composite allograft tolerance induction and maintenance. Transplantation. 2021;105:1238–1249. doi: 10.1097/TP.0000000000003509. [DOI] [PubMed] [Google Scholar]
- 24.Shi M., Liu Z., Wang Y., Xu R., Sun Y., Zhang M., et al. A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med. 2017;6:2053–2061. doi: 10.1002/sctm.17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X.L., Nakamura S., Li Q., Wysoczynski M., Gumpert A.M., Wu W.J., et al. Repeated administrations of cardiac progenitor cells are superior to a single administration of an equivalent cumulative dose. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolli R. Repeated cell therapy: a paradigm shift whose time has come. Circ Res. 2017;120:1072–1074. doi: 10.1161/CIRCRESAHA.117.310710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plock J.A., Schnider J.T., Schweizer R., Zhang W., Tsuji W., Waldner M., et al. The influence of timing and frequency of adipose-derived mesenchymal stem cell therapy on immunomodulation outcomes after vascularized composite allotransplantation. Transplantation. 2017;101:e1–e11. doi: 10.1097/TP.0000000000001498. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez A.E., Cheng H.Y., Lao W.W., Wang Y.L., Wen C.J., Wallace C.G., et al. A novel rat full-thickness hemi-abdominal wall/hindlimb osteomyocutaneous combined flap: influence of allograft mass and vascularized bone marrow content on vascularized composite allograft survival. Transpl Int. 2014;27:977–986. doi: 10.1111/tri.12364. [DOI] [PubMed] [Google Scholar]
- 29.Cheng H.Y., Ghetu N., Huang W.C., Wang Y.L., Wallace C.G., Wen C.J., et al. Syngeneic adipose-derived stem cells with short-term immunosuppression induce vascularized composite allotransplantation tolerance in rats. Cytotherapy. 2014;16:369–380. doi: 10.1016/j.jcyt.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Macedo C., Tran L.M., Zahorchak A.F., Dai H., Gu X., Ravichandran R., et al. Donor-derived regulatory dendritic cell infusion results in host cell cross-dressing and T cell subset changes in prospective living donor liver transplant recipients. Am J Transplant. 2021;21:2372–2386. doi: 10.1111/ajt.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo Y.R., Chen C.C., Chen Y.C., Chien C.M. Recipient adipose-derived stem cells enhance recipient cell engraftment and prolong allotransplant survival in a miniature swine hind-limb model. Cell Transplant. 2017;26:1418–1427. doi: 10.1177/0963689717724534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Bene M., Di Caprio A.P., Melzi M.L., Pioltelli P.E., Bonomi S. Autologous mesenchymal stem cells as a new strategy in immunosuppressant therapy in double hand allotransplantation. Plast Reconstr Surg. 2013;131:305e–307e. doi: 10.1097/PRS.0b013e318278d648. [DOI] [PubMed] [Google Scholar]
- 33.Jang Y., Koh Y.G., Choi Y.J., Kim S.H., Yoon D.S., Lee M., et al. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. In Vitro Cell Dev Biol Anim. 2015;51:142–150. doi: 10.1007/s11626-014-9814-6. [DOI] [PubMed] [Google Scholar]
- 34.Taguchi T., Borjesson D.L., Osmond C., Griffon D.J. Influence of donor's age on immunomodulatory properties of canine adipose tissue-derived mesenchymal stem cells. Stem Cell Dev. 2019;28:1562–1571. doi: 10.1089/scd.2019.0118. [DOI] [PubMed] [Google Scholar]
- 35.Liu H., Wang Y., Zeng Q., Zeng Y.Q., Liang C.L., Qiu F., et al. Suppression of allograft rejection by CD8+CD122+PD-1+ Tregs is dictated by their Fas ligand-initiated killing of effector T cells versus Fas-mediated own apoptosis. Oncotarget. 2017;8:24187–24195. doi: 10.18632/oncotarget.15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noyan F., Zimmermann K., Hardtke-Wolenski M., Knoefel A., Schulde E., Geffers R., et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am J Transplant. 2017;17:917–930. doi: 10.1111/ajt.14175. [DOI] [PubMed] [Google Scholar]
- 37.Jhunjhunwala S., Balmert S.C., Raimondi G., Dons E., Nichols E.E., Thomson A.W., et al. Controlled release formulations of IL-2, TGF-β1 and rapamycin for the induction of regulatory T cells. J Contr Release. 2012;159:78–84. doi: 10.1016/j.jconrel.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher J.D., Balmert S.C., Zhang W., Schweizer R., Schnider J.T., Komatsu C., et al. Treg-inducing microparticles promote donor-specific tolerance in experimental vascularized composite allotransplantation. Proc Natl Acad Sci U S A. 2019;116:25784–25789. doi: 10.1073/pnas.1910701116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng H.Y., Tay S.K.L., Wen C.J., Lin C.F., Wang A.Y., Shih L.Y., et al. Bioimaging of alloantigen-stimulated regulatory T cells in rat vascularized composite allotransplantation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchinson J.A., Riquelme P., Sawitzki B., Tomiuk S., Miqueu P., Zuhayra M., et al. Cutting Edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187:2072–2078. doi: 10.4049/jimmunol.1100762. [DOI] [PubMed] [Google Scholar]
- 42.Radu C.A., Horn D., Kiefer J., Rebel M., Gebhard M.M., Ryssel H., et al. Donor-derived transplant acceptance-inducing cells in composite tissue allotransplantation. J Plast Reconstr Aesthetic Surg. 2012;65:1684–1691. doi: 10.1016/j.bjps.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Tsang J.Y., Tanriver Y., Jiang S., Xue S.A., Ratnasothy K., Chen D., et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez-Fueyo A., Whitehouse G., Grageda N., Cramp M.E., Lim T.Y., Romano M., et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20:1125–1136. doi: 10.1111/ajt.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furlan S.N., Singh K., Lopez C., Tkachev V., Hunt D.J., Hibbard J., et al. IL-2 enhances ex vivo-expanded regulatory T-cell persistence after adoptive transfer. Blood Adv. 2020;4:1594–1605. doi: 10.1182/bloodadvances.2019001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratnasothy K., Jacob J., Tung S., Boardman D., Lechler R.I., Sanchez-Fueyo A., et al. IL-2 therapy preferentially expands adoptively transferred donor-specific Tregs improving skin allograft survival. Am J Transplant. 2019;19:2092–2100. doi: 10.1111/ajt.15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yolcu E.S., Shirwan H., Askenasy N. Mechanisms of tolerance induction by hematopoietic chimerism: the immune perspective. Stem Cells Transl Med. 2017;6:700–712. doi: 10.1002/sctm.16-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulahci Y., Siemionow M. A new composite hemiface/mandible/tongue transplantation model in rats. Ann Plast Surg. 2010;64:114–121. doi: 10.1097/SAP.0b013e3181a20cca. [DOI] [PubMed] [Google Scholar]
- 49.Kulahci Y., Klimczak A., Madajka M., Altuntas S., Siemionow M. Long-term survival of composite hemiface/mandible/tongue allografts correlates with multilineage chimerism development in the lymphoid and myeloid compartments of recipients. Transplantation. 2010;90:843–852. doi: 10.1097/TP.0b013e3181f28bb0. [DOI] [PubMed] [Google Scholar]
- 50.Wekerle T., Kurtz J., Ito H., Ronquillo J.V., Dong V., Zhao G., et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 51.Miller J.F.A.P. The function of the thymus and its impact on modern medicine. Science. 2020;369 doi: 10.1126/science.aba2429. [DOI] [PubMed] [Google Scholar]
- 52.Siemionow M., Izycki D., Ozer K., Ozmen S., Klimczak A. Role of thymus in operational tolerance induction in limb allograft transplant model. Transplantation. 2006;81:1568–1576. doi: 10.1097/01.tp.0000209508.37345.82. [DOI] [PubMed] [Google Scholar]
- 53.Bozkurt M., Klimczak A., Nasir S., Zor F., Krokowicz L., Siemionow M. Composite osseomusculocutaneous sternum, ribs, thymus, pectoralis muscles, and skin allotransplantation model of bone marrow transplantation. Microsurgery. 2013;33:43–50. doi: 10.1002/micr.22023. [DOI] [PubMed] [Google Scholar]
- 54.Zor F., Bozkurt M., Cwykiel J., Karagoz H., Kulahci Y., Uygur S., et al. The effect of thymus transplantation on donor-specific chimerism in the rat model of composite osseomusculocutaneous sternum, ribs, thymus, pectoralis muscles, and skin allotransplantation. Microsurgery. 2020;40:576–584. doi: 10.1002/micr.30555. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q., Zhang M., Ding G., Liu Y., Sun Y., Wang J., et al. Anti-ICAM-1 antibody and CTLA-4Ig synergistically enhance immature dendritic cells to induce donor-specific immune tolerance in vivo. Immunol Lett. 2003;90:33–42. doi: 10.1016/s0165-2478(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 56.Pons J.A., Revilla-Nuin B., Baroja-Mazo A., Ramirez P., Martinez-Alarcon L., Sanchez-Bueno F., et al. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation. 2008;86:1370–1378. doi: 10.1097/TP.0b013e318188d3e6. [DOI] [PubMed] [Google Scholar]
- 57.Kawai T., Sachs D.H., Sykes M., Cosimi A.B. Immune Tolerance N: HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013;368:1850–1852. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., Zheng Z., Wang Y., Liu J., Li N., Hu X., et al. Role of donor-specific regulatory T cells in long-term acceptance of rat hind limb allograft. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell D.J. Control of regulatory T cell migration, function, and homeostasis. J Immunol. 2015;195:2507–2513. doi: 10.4049/jimmunol.1500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomura M., Honda T., Tanizaki H., Otsuka A., Egawa G., Tokura Y., et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest. 2010;120:883–893. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mailloux A.W., Young M.R. NK-dependent increases in CCL22 secretion selectively recruits regulatory T cells to the tumor microenvironment. J Immunol. 2009;182:2753–2765. doi: 10.4049/jimmunol.0801124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marshall L.A., Marubayashi S., Jorapur A., Jacobson S., Zibinsky M., Robles O., et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fisher J.D., Zhang W., Balmert S.C., Aral A.M., Acharya A.P., Kulahci Y., et al. In situ recruitment of regulatory T cells promotes donor-specific tolerance in vascularized composite allotransplantation. Sci Adv. 2020;6 doi: 10.1126/sciadv.aax8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brazio P.S., Munivenkatappa R.B., Bojovic B., Ha J.S., Brown E.N., Hess A.S., et al. Regulatory T cells are not predictive of outcomes in a nonhuman primate model of vascularized composite allotransplantation. Transplantation. 2013;96:267–273. doi: 10.1097/TP.0b013e318298dcff. [DOI] [PubMed] [Google Scholar]
- 65.Ezzelarab M.B., Zhang H., Guo H., Lu L., Zahorchak A.F., Wiseman R.W., et al. Regulatory T cell infusion can enhance memory T cell and alloantibody responses in lymphodepleted nonhuman primate heart allograft recipients. Am J Transplant. 2016;16:1999–2015. doi: 10.1111/ajt.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eljaafari A., Badet L., Kanitakis J., Ferrand C., Farre A., Petruzzo P., et al. Isolation of regulatory T cells in the skin of a human hand-allograft, up to six years posttransplantation. Transplantation. 2006;82:1764–1768. doi: 10.1097/01.tp.0000250937.46187.ca. [DOI] [PubMed] [Google Scholar]
- 67.Lin C.H., Zhang W., Ng T.W., Zhang D., Jiang J., Pulikkottil B., et al. Vascularized osteomyocutaneous allografts are permissive to tolerance by induction-based immunomodulatory therapy. Am J Transplant. 2013;13:2161–2168. doi: 10.1111/ajt.12275. [DOI] [PubMed] [Google Scholar]
- 68.Ortiz L.A., Dutreil M., Fattman C., Pandey A.C., Torres G., Go K., et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nasef A., Chapel A., Mazurier C., Bouchet S., Lopez M., Mathieu N., et al. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13:217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuo Y.R., Chen C.C., Goto S., Huang Y.T., Wang C.T., Tsai C.C., et al. Immunomodulatory effects of bone marrow-derived mesenchymal stem cells in a swine hemi-facial allotransplantation model. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitsuzawa S., Ikeguchi R., Aoyama T., Ando M., Takeuchi H., Yurie H., et al. Induced pluripotent stem cell-derived mesenchymal stem cells prolong hind limb survival in a rat vascularized composite allotransplantation model. Microsurgery. 2019;39:737–747. doi: 10.1002/micr.30507. [DOI] [PubMed] [Google Scholar]
- 72.Jeong S.H., Ji Y.H., Yoon E.S. Immunosuppressive activity of adipose tissue-derived mesenchymal stem cells in a rat model of hind limb allotransplantation. Transplant Proc. 2014;46:1606–1614. doi: 10.1016/j.transproceed.2013.12.069. [DOI] [PubMed] [Google Scholar]
- 73.Kuo Y.R., Chen C.C., Goto S., Lee I.T., Huang C.W., Tsai C.C., et al. Modulation of immune response and T-cell regulation by donor adipose-derived stem cells in a rodent hind-limb allotransplant model. Plast Reconstr Surg. 2011;128:661e–672e. doi: 10.1097/PRS.0b013e318230c60b. [DOI] [PubMed] [Google Scholar]
- 74.Lavorato A., Raimondo S., Boido M., Muratori L., Durante G., Cofano F., et al. Mesenchymal stem cell treatment perspectives in peripheral nerve regeneration: systematic review. Int J Mol Sci. 2021;22:572. doi: 10.3390/ijms22020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marin E., Cuturi M.C., Moreau A. Tolerogenic dendritic cells in solid organ transplantation: where do we stand? Front Immunol. 2018;9:274. doi: 10.3389/fimmu.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Detry O., Vandermeulen M., Delbouille M.H., Somja J., Bletard N., Briquet A., et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: a phase I-II, open-label, clinical study. J Hepatol. 2017;67:47–55. doi: 10.1016/j.jhep.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Rocamora-Reverte L., Melzer F.L., Wurzner R., Weinberger B. The complex role of regulatory T cells in immunity and aging. Front Immunol. 2020;11:616949. doi: 10.3389/fimmu.2020.616949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathew J.M., HV J., LeFever A., Konieczna I., Stratton C., He J., et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep. 2018;8:7428. doi: 10.1038/s41598-018-25574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savage T.M., Shonts B.A., Obradovic A., Dewolf S., Lau S., Zuber J., et al. Early expansion of donor-specific Tregs in tolerant kidney transplant recipients. JCI Insight. 2018;3 doi: 10.1172/jci.insight.124086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fryer M., Grahammer J., Khalifian S., Furtmüller G.J., Lee W.P., Raimondi G., et al. Exploring cell-based tolerance strategies for hand and face transplantation. Expet Rev Clin Immunol. 2015;11:1189–1204. doi: 10.1586/1744666X.2015.1078729. [DOI] [PubMed] [Google Scholar]
- 81.Leonard D.A., Kurtz J.M., Mallard C., Albritton A., Duran-Struuck R., Farkash E.A., et al. Vascularized composite allograft tolerance across MHC barriers in a large animal model. Am J Transplant. 2014;14:343–355. doi: 10.1111/ajt.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schweizer R., Taddeo A., Waldner M., Klein H.J., Fuchs N., Kamat P., et al. Adipose-derived stromal cell therapy combined with a short course nonmyeloablative conditioning promotes long-term graft tolerance in vascularized composite allotransplantation. Am J Transplant. 2020;20:1272–1284. doi: 10.1111/ajt.15726. [DOI] [PubMed] [Google Scholar]
- 83.Todo S., Yamashita K., Goto R., Zaitsu M., Nagatsu A., Oura T., et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632–643. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 84.Sawitzki B., Harden P.N., Reinke P., Moreau A., Hutchinson J.A., Game D.S., et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet. 2020;395:1627–1639. doi: 10.1016/S0140-6736(20)30167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roemhild A., Otto N.M., Moll G., Abou-El-Enein M., Kaiser D., Bold G., et al. Regulatory T cells for minimizing immune suppression in kidney transplantation: phase I/IIa clinical trial. BMJ. 2020;371:m3734. doi: 10.1136/bmj.m3734. [DOI] [PMC free article] [PubMed] [Google Scholar]