Abstract
In the last 10– 15 years, there has been a recognition that the catecholamines (norepinephrine, NE, and epinephrine, Epi) released by the sympathetic nervous system under stressful conditions promote tumor growth through a variety of mechanisms. Tumors recruit autonomic nerves during their development and NE is then released locally in the tumor microenvironment (TME). Acting through adrenergic receptors present on a variety of cells in the TME, NE and Epi induce proliferation, resistance to apoptosis, epithelial to mesenchymal transition, metastasis of tumor cells, angiogenesis, and inflammation in the TME. These pre-clinical studies have been conducted in mouse models whose care and housing parameters are outlined in “The Guide for the Care and Use of Laboratory Animals [1]. In particular, the Guide mandates that mice be housed at standardized sub-thermoneutral temperatures; however, this causes a state of chronic cold-stress and elevated levels of NE. Although mice are able to maintain a normal body temperature when kept at these cool temperatures, it is becoming clear that this cold-stress is sufficient to activate physiological changes which affect experimental outcomes. We find that when mice are housed under standard, sub-thermoneutral temperatures (~22°C, ST), tumor growth is significantly greater than when mice are housed at thermoneutrality (~30°C TT). We also find that the anti-tumor immune response is suppressed at ST and this immunosuppression can be reversed by housing mice at TT or by administration of propranolol (a β-adrenergic receptor antagonist) to mice housed at ST. Furthermore, at ST tumors are more resistant to therapy and can also be sensitized to cytotoxic therapies by housing mice at TT or by treating mice with propranolol. The implications of these observations are particularly relevant to the way in which experiments conducted in preclinical models are interpreted and the findings implemented in the clinic. It may be that the disappointing failure of many new therapies to fulfill their promise in the clinic is related to an incomplete preclinical assessment in mouse models. Further, an expanded understanding of the efficacy of a therapy alone or in combination obtained by testing under a wider range of conditions would better predict how patients, who are under various levels of stress, might respond in a clinical setting. This may be particularly important to consider since we now appreciate that long term outcomes of many therapies depends on eliciting an immune response.
It is clear that the outcome of metabolic experiments, immunological investigations and therapeutic efficacy testing in tumors of mice housed at ST is restricted and expanding these experiments to include results obtained at TT may provide us with valuable information that would otherwise be overlooked.
Keywords: Metabolism, Tumor microenvironment, Thermoregulation, Therapeutic resistance, Immunosuppression
Introduction:
The TME regulates tumor growth and response to therapy in many ways. Recently, it has been shown that tumors recruit both sympathetic and parasympathetic nerves which produce norepinephrine and acetylcholine in the TME, promoting tumorigeneses, invasion and metastasis [2, 3]. In addition to acting directly on tumor cells, norepinephrine (NE) can regulate the activity of immune cells. The regulation of immune cells is complex; in addition to the cytokines/chemokines released from other cells, they are also responsive to signals from the nervous system. In fact, both primary and secondary immune organs are densely innervated by fibers of the sympathetic nervous system [4]so that the major pathway by which the nervous system controls the immune system is by local release of the neurotransmitter NE from post-ganglionic sympathetic neurons in various immune organs [4, 5]. This pathway is activated during the sympathetic stress response and although in response to an acute stress, sympathetic activation of immune cells is beneficial, when this stress is chronic, there is much evidence that the sympathetic nervous system suppresses immune responses. How is this relevant to pre-clinical mouse models used for research? Lately, concern has been raised that experimental mice in standard housing conditions are “metabolically morbid” [6] and under constant cold stress [7–16]. Our group has observed different biological outcomes in preclinical mouse models of cancer and tumor immunity between mice that are cold-stressed and those in which cold stress is reduced, even though the core body temperature in both groups is the same. An incomplete recognition of these potential differences in experimental outcome could significantly limit the full potential of preclinical models of cancer and other diseases. Here, we will present an overview of this problem with special focus on how housing conditions subject laboratory mice to chronic cold stress, resulting in elevated norepinephrine levels, and the suppressive effects of this increased adrenergic signaling on the anti-tumor immune response and tumor response to therapy.
1. Metabolic effects of “shoe-box” caging on experimental mice
Mice have become the most widely used models for studying human/patient biological processes including development, metabolism, normal physiology and disease. “The Guide for the Care and Use of Laboratory Animals” [1] provides detailed guidelines for all aspects of laboratory mouse housing and is followed by research institutions internationally. Comprehensive parameters are provided for all environmental factors including temperature, humidity, ventilation, food, lighting, noise and cage size/ housing density as well as recommendations for enrichment strategies that can reduce stress. It is stated several times in different places that variations in these microenvironmental factors could affect behavior, physiology (reproduction), phenotype and, possibly, experimental outcomes. These recommendations are based on data from publications and experts, being a synthesis of all empirical aspects of operating the animal facilities and are revised periodically (the last edition was in 2011). In practice, animal care personnel handle implementation of these regulations and therefore, the majority of scientists do not take these environmental variables into consideration when designing experiments and analyzing experimental outcomes. They assume the mice are healthy and the outcomes of experiments routinely conducted under these mandated conditions will provide accurate and reproducible baseline data. However, recently, a growing number of investigators have raised significant concerns that this may not be the case.
The first contemporary warning was published by Martin et al, a group at the National Institute of Aging [6]. These investigators raised the alarm by pointing out that, contrary to these presumptions of health, “mice under standard conditions are sedentary, overfed, obese, glucose intolerant” and hypertensive. More importantly, they warned that the biological status of these mice likely “confounds data interpretation on outcomes of human studies”. These standard control animals are also at higher risk for developing cancer, diabetes, renal failure and premature death than mice which have reduced food intake, exercise more and have a stimulating environment. In fact, reducing caloric intake can increase life span up to 40% and this is largely due to reduction in these diseases. In a genomic study, this group found significant differences in gene expression when rats on different diets were compared with standard vs lean controls, again emphasizing that the metabolic condition of the control animals has the capability of skewing the results of experiments. Furthermore, they discuss studies suggesting that the efficacy of drugs for treating metabolic, neurological and malignant disease may be more efficacious in mice housed under standard conditions than in more healthy mice, thus contributing to the failure of several drugs to recapitulate the success seen in preclinical models when these drugs are used with patients. They conclude that “The beneficial effects of some drugs in animal models might result from their effects on processes associated with an unhealthy lifestyle (increased oxidative stress, inflammation, insulin resistance, etc.) rather than a specific effect of the drug on the disease process” and propose that experiments should be designed to include both sets of conditions rather than just the one standard one. Other biological concerns about laboratory mice are also being raised. For instance, the immune system which develops in laboratory mice housed under extremely clean conditions is significantly different than that of feral mice which more closely resembles the immune system of humans and this can be altered by exposure to feral mice indicating another environmental variable that can significantly affect experimental outcomes [17].
2. Effects of housing temperature on mice: differences between mice at ST and TT
Whereas, Martin et al [6] were concerned about the metabolic effects of a sedentary, obesogenic lifestyle, a housing parameter which we and others have recently become particularly concerned about is the ambient temperature at which mice are housed. Biologists have studied thermoregulation in mice for years, and are well aware of the unique aspects of their physiology with regard to body temperature control, but it is now becoming clear that the variable of housing temperature has a significant impact on many aspects of mouse physiology which directly affect experimental outcomes [7, 12–14, 18–39]. This is because mice have a large surface to volume ratio and therefore lose heat more quickly in cool temperatures [12, 18]. Of particular concern is the fact that the temperature range recommended by the Guide[1], between 22–26°C, is below the resting metabolic thermoneutral zone of the mouse[18]. This thermoneutral zone is defined as the ambient temperature range at which a stable core temperature is achieved by “adjustments in insulation, posture, and skin blood flow” and is 30–32°C for mice [18, 40]. In other words, the animal is able to maintain core temperature by basal metabolism alone without activating physiological, thermoregulatory processes for heat production or heat loss which require large amounts of energy. It has been shown that mice, when given a choice, will choose an ambient temperature of 30.9°C from a range of 18– 34C [41]. Gordon states that the Lower Critical Temperature (LCT) has been extensively studied, is approximately 30°C and is the point at which mice become susceptible to cold stress. Although mice will select a temperature a few degrees lower during their active, nocturnal period, their core temperature is maintained by heat produced through increased activity. “The Guide” acknowledges that the recommended temperature is lower than thermoneutrality, but it specifically recommends that housing temperatures be kept below the animal’s lower critical temperature to avoid heat stress. To compensate, mice often huddle together and although “The Guide” suggests that mice can be given nesting materials and shelters, see also [42, 43]; this is often not done and, therefore there is great potential for laboratory mice to be subjected to chronic cold stress. The reason this situation has overall not worried investigators, though, is because mice are able to effectively thermoregulate and the core temperatures at standard temperatures and thermoneutral temperatures are not significantly different [41].
How does this chronic cold stress affect laboratory mice and the outcomes of experiments? There are clear differences in the metabolism of mice housed at standard temperature (ST- 22–26°C) and those housed at thermoneutral temperatures (TT- 30–32°C) as reviewed by Overton[12]. Although the core body temperatures of mice may vary by 2 degrees during the course of a day, in concert with circadian rhythm and activity level, the core temperature is similar between mice housed at ST and TT [18, 24, 41]. Therefore, the physiological differences are related to increased metabolism and thermogenesis at ST which are required to defend core body temperature. Uchida et al [34] conducted a study comparing glucose homeostasis in C57BL/6 mice housed at 25°C v 20°C (instead of the 4°C which is commonly used to study cold stress). Interestingly, there was no difference in blood glucose or plasma insulin levels in mice, however fasting levels differed significantly with lower insulin and higher glucose levels at the lower temperature. This correlated with an impaired response in a glucose tolerance test. These authors found a significant impairment of glucose-induced insulin secretion (comparable to that seen at 4°C), which resulted in elevated glucose levels (unlike the response at 4°C). Additionally, when 20°C mice were moved back to 25°C, they reverted to the normal phenotype. They also found that the 20°C mice had elevated plasma NE but not Epi. NE is known to inhibit insulin secretion from the pancreatic islets [44] and is the stress hormone which drives thermogenesis to maintain body temperature. In measuring NE turnover in various organs, Teramura and colleagues found that the rate of NE turnover and upregulation of UCP-1 in BAT was similar whether the mice were at 4°C or at 23°C [45] confirming that physiologically, the degree of cold stress experienced at ST is comparable to that experienced in classic “cold stress” experiments. Comparison of skin temperatures at ST and TT found lower skin temperature in the 20°C mice while confirming there was no difference between the core temperatures in the two groups. Lastly, these authors found that changes in the cool mice related to lipid metabolism and fat storage. Clearly, differences in energy metabolism occur at these two sub-thermoneutral temperatures and it would be interesting to compare these results with those from mice housed at TT. These metabolic differences are mirrored by differences in heart rate and blood pressure. Swoap and colleagues have shown that as the ambient housing temperature decreases, heart rate and blood pressure significantly increase [30, 31]. The resting heart rate at 22°C is 550–600 bpm while at 30°C, it is reduced to 350–400 bpm [12, 30]}. In fact, although it was thought that the autonomic control of heart rate differed between mice and men, these authors concluded that when the autonomic control of heart rate is studied in animals at TT, it is controlled by parasympathetic vagal input in a manner similar to humans, rather than by sympathetic inputs that prevail at ST. These discrepancies call attention to the need to consider ambient temperature when conducting cardiovascular experiments in mice and relating results to humans.
The validity of these warnings about consideration of ambient housing temperature in assessing results from mouse models is clearly demonstrated in experiments with the UCP-1 knock out mouse. UCP-1 is the “uncoupling protein-1” of mitochondrial inner membrane in brown adipose tissue (BAT) which mediates a thermogenic proton leak, uncoupling oxidative phosphorylation from ATP production and thereby dissipating energy and generating heat by non-shivering thermogenesis in BAT. In one experiment a UCP-1 knock-out mouse developed the expected deficits in non-shivering thermogenesis, but it did not become obese as was expected [46, 47]. This cast doubt on the involvement of UCP-1 in bioenergetics and the usefulness of targeting it to combat obesity. However, these mice were housed at ST and more recently, several groups have shown that these mice do become obese if they are housed at TT [7, 47–49] suggesting that in UCP-1 −/− mice, alternative pathways must exist for thermogenesis which burns calories to generate body heat and prevents obesity at ST. A commentary accompanying the Feldman paper reinforced the fact that ambient temperature is a critical variable to consider when assessing the effects of different genotypes in metabolic research [9]. Interestingly, a recent study of the anti-obesity efficacy of 2,4-dinitrophenol (DNP, a chemical uncoupler) concluded that in experiments conducted at TT, DNP treatment decreased body fat by 26% and improved glucose tolerance, but no beneficial effects were observed at ST [50]. This group also tested the β3-adrenergic agonist, CL316243, to determine whether pharmacological activation of brown adipose tissue (which is the major tissue expressing β3-AR) could result in weight loss; again they observed beneficial effects at TT, but not at ST [14]. Ravussin, commenting on the Feldman paper, takes the position that “ambient temperature clearly affects phenotypes related to energy homeostasis in rodents” [51]. Related to the increased metabolism seen in mice at ST vs TT, Jun el al found that mice at ST had increased lipid uptake in BAT, heart, and lungs and that hypoxia, by suppressing metabolism, caused increased levels of triglycerides in the plasma; however, when mice were exposed to hypoxic conditions at TT, no differences in plasma tryglycerides were detected [52]. One study found differences in the effects of energy restriction on the disease progression of lymphoma over the course of the lives of C57BL/6 mice fed an energy restricted diet at either ST or TT. At ST these mice lived significantly longer than either control mice at ST or mice on an energy restricted diet at TT [53].
3. Effects of housing temperature on mouse models of infection
There are many studies reporting the deleterious effects of stressors such as restraint and social isolation on the immune response in infection models at standard room temperatures [54, 55]. However, the immune response is also profoundly affected by housing temperature. One hallmark of an effective immune response is the “fever” response in which the set-point of the core temperature is elevated and the organism recruits thermogenic mechanisms to raise the body temperature. It was thought that mice do not generate fevers as humans do, but it was recently shown that although mice fail to develop a fever following injection of LPS at ST, fevers are generated when they are challenged at thermoneutrality [27]. Are other aspects of the immune response affected by housing temperature? It is known that immune organs are heavily innervated by sympathetic nerve fibers [4] while immune cells express adrenergic receptors, primarily β2-ARs, in a cell type/subset specific pattern [56]. In terms of the overall effect of stress on the immune response, the effect depends on whether the stress is acute (of short duration) or chronic. During the “fight or flight” acute stress response, the immune response is mobilized by sympathetic signaling. This has been hypothesized to be a key evolutionary mechanism by which animals survive stressful challenges which likely would involve injury or exposure to pathogens [57]. Generally speaking, acute stress is “beneficial”, mobilizing immune cells to the site and promoting their protective function, while, in contrast, chronic stress is “detrimental” and leads to systemic immunosuppression[54, 57]. As discussed above, laboratory mice housed at ST are chronically cold stressed and thus have elevated NE levels associated with thermogenesis. A relationship between room temperature and the course of pathogenic infections was reported 70 yrs. ago when Moragues noticed that dramatic differences in disease progression, severity and survival following infection with murine typhus rickettsiae correlated with seasonal differences in room temperature, in that all the mice died of disease when the room temperature was approximately 18–23°C while few deaths occurred when the room was 29–37°C [58]. Similarly, mice infected with Coe virus had markedly better survival when held at 36°C vs. 25 °C [59]. A more recent study emphasizes the fact that normal mice housed at 22, 26 or 30 °C all are able to maintain a normal core temperature, which as expected, cycles between 35.5 and 37.5 °C with circadian rhythm [23]. In this study, mice were infected with influenza virus and housed at the three temperatures; the mice at 30 °C showed less “sick behavior” (sleep disturbances, reduced locomotion, inflammatory cytokines) than the mice at the lower temperatures [23]. These studies serve to illustrate the detrimental effects of ST on immune responses to pathogens. Interestingly, there are reports that β-adrenergic blockade (i.e. with propranolol) is able to improve outcomes in viral [60] and parasitic [61, 62] infections in mice housed at ST. This suggests that blocking NE β-adrenergic signaling in these models is the underlying mechanism of the beneficial effect. A study by Grebe et al [63], in C57/BL6 mice infected with influenza A virus showed that administration of a β2-AR antagonist enhanced the anti-viral responses of CD8+ T-cells (IFNγ expression). Again, it would be interesting to compare the benefit of β2-AR blockade in experiments such as these done at ST with experiments done at TT to determine whether there would be any benefit when NE levels are ameliorated by thermoneutral housing.
4. Thermoneutrality vs. Hyperthermia Treatment (Thermal therapy)
In another early study, the effect of ambient temperatures of 20–22 °C vs 35 °C on rabies infected mice was investigated and it was found that the survival rate of mice housed at 35 °C was significantly higher [64]. However, the core temperatures of mice housed at 35 °C were higher than normal (39.5°C) so that these mice were actually experiencing hyperthermia resulting from the very warm ambient temperatures in which they were housed. Our lab, and many others, has shown that the stress of a short mild hyperthermia treatment can boost immune responses, including anti-tumor activity [65]. The distinction between the thermal/physiological effects of housing mice at thermoneutrality (30–32°C) and thus exposing mice to temperatures high enough to raise the core temperature is an important one. At TT, mice are able to maintain a normal body temperature of ~37 °C [23, 41] via basal metabolism and do not need to expend energy to warm or cool themselves. On the other hand, the goal of many preclinical studies for “thermal therapy” or “hyperthermia” is to expose mice to a temperature high enough to raise the tumor temperature, or core body temperature several degrees, which has been observed to alter the tumor microenvironment, reduce interstitial fluid pressure, [66] improve efficacy of radiation and chemotherapy [67] and may trigger various molecular thermostats that are similar to those activated by a fever, helping to boost the immune system [65, 68].
In this active field of hyperthermia research, investigators are well aware of the beneficial effect that short exposures to a warm environmental temperature can have on immune cell activity. However, even in this research field, no studies have examined whether these differences result from the fact that control mice are cold stressed compared to mice in which core temperatures are elevated. It is clear that even research designed to determine the impact of temperature shifts locally or systemically in terms of improving cancer treatment may be (unbeknownst to the investigators) influenced by cold stress in control groups. In this regard, it will be interesting to see the degree of beneficial effects of hyperthermia treatments in mice housed mice at TT.
5. Adrenergic signaling and tumor growth at ST
How does the fact that mice at ST are chronically cold-stressed and have elevated NE levels compared to mice at TT impact tumor growth? β-adrenergic receptors (β-ARs) are found on immune cells and are on many tumor cells [69]. Emerging evidence from experiments conducted at ST links catecholamines to tumor progression and this topic has been recently reviewed [70–72]. Evidence for the pro-tumorigenic role of adrenergic signaling comes from both epidemiological studies and experiments with preclinical mouse models. Retrospective analyses by several groups in different tumor types support the idea that patients who were taking β-adrenergic antagonists (β-blockers) for non-cancer indications had reduced disease progression and/or better survival in breast [73–75], ovarian [76, 77]melanoma [78], lung [79], prostate [80], pancreatic [81], cancers. However a few studies have not found benefit [82–85] Interestingly, Lutgendorf et al found, in ovarian cancer patients, that higher NE levels in the tumors correlated with more advanced disease and the degree of social stress experienced by the patients[86]. Experimental evidence showed that adrenergic signaling induced migratory behavior in tumor cells in vitro (e.g. SW480 human colon carcinoma cells) which could be inhibited by β2-AR blockade [87] and that while treatment of mice with NE increased the development of lymph node metastases (PC-3 prostate cancer cells), this could also be prevented by β2-AR blockade with propranolol [88]. Le et al [89] have more recently investigated this phenomenon and found that adrenergic signaling recruited inflammatory macrophages to the TME and these induced VEGFC expression by tumors, which leads to remodeling of lymphatics and metastatic spread of breast cancer in a mouse model. In a retrospective patient study, this group found evidence that β-blockers significantly reduced lymph node metastases in patients [89]. In a model of social stress, Hasegawa showed that the stress enhanced fibrosarcoma growth promotion could be inhibited with propranolol [90]. β-adrenergic signaling induces tumor cell proliferation [91, 92], invasion [93, 94], protection from anoikis [95], metastasis [94, 96, 97]and changes in the tumor microenvironment such as angiogenesis [98–100]. Thaker et al used restraint stress or social isolation to show that chronic stress increases catecholamine (NE and epinephrine) levels, increases VEGF and vascularization and increases tumor growth [101]. These effects could be mimicked by treatments with specific β2-AR agonists and reversed by β-AR blockers. Epinephrine also protects prostate cancer cells from apoptosis [102] through phosphorylation of BAD. A role for this anti-apoptotic pathway was demonstrated in prostate cancer models in which restraint stress protected xenografts from apoptosis induced by a PI3K inhibitor by induction of BAD phosphorylation and again, this effect could be blocked by a β2-AR specific antagonist [103]. There are many other examples of stress induced tumor growth (e.g. [104]). The anatomical basis for adrenergic signaling in the tumor microenvironment was clarified by the work of Magnon and Frenette who were able to visualize both sympathetic and parasympathetic fibers in prostate tumors in mice and show that sympathectomy (preventing the release of NE in the TME) prevented early aspects of cancer development while parasympathetic signaling promoted invasion and metastasis [2]. These authors thus demonstrated that tumors actively recruit autonomic innervation by neurogenesis to support growth in a process akin to angiogenesis (see also [105]). A recent study investigated the possible benefits of combining propranolol and chemotherapy. In a mouse breast cancer model (MDA-MB-231 human cell line in nude mice), Pasquier et al found that at the very effective doses of chemotherapy used, propranolol did not significantly improve the anti-tumor efficacy, however the median survival was significantly enhanced [106]. These authors also demonstrated that propranolol did, however, enhance the anti-angiogenic effects of chemotherapy in vitro. Together, the epidemiological studies suggesting clinical benefit of β-blockers to cancer patients and the compelling pre-clinical data defining the tumor promoting effects of adrenergic signaling provide enthusiasm and a strong rationale for testing the anti-cancer efficacy of β-blockers in clinical trials in combination with other therapies.
6. The anti-tumor immune response and response to therapeutics is significantly improved by housing at thermoneutrality or β-adrenergic receptor blockade at standard housing temperatures.
In investigating the effects of cold-stress, researchers have taken mice acclimated to standard housing temperatures and subjected them to much lower temperatures (4°C). However, it is clear from the studies discussed above, that mice at ST are already living with chronic cold stress and the turnover of NE in mice at 4°C and 22°C is not significantly different [45]. Therefore, compared to TT, all studies of tumor growth have been conducted under some degree of cold stress and studies of the effect of any stress on tumor growth are actually studies of exacerbated stress.
How is tumor growth affected if adrenergic cold stress in mice is alleviated by housing mice at thermoneutrality? We have previously reported that tumor growth in several syngeneic murine tumor models is significantly reduced when tumor bearing mice are housed at 30°C instead of 22°C [24]. In these experiments, mice were acclimated to ST or TT for 1–3 weeks prior to tumor implantation; we also used moderate numbers of tumor cells to allow for development of an effective anti-tumor immune response rather than the higher numbers that are often used to insure rapid tumor growth. These models included 4T1 mammary tumors and CT26 colon adenocarcinomas in BALB/c mice and B16.F10 melanoma and Pan02 in C57BL/6 mice, as well as MCA carcinogen induced tumors in BALB/c mice. Additionally, we observed that spontaneous lung metastases of 4T1 to the lungs were also significantly reduced at TT. When these same tumor models were grown in immunodeficient SCID or nude mice, no difference in growth occurred. This points to a critical role for the adaptive immune response in this improved tumor control at TT and this is confirmed by experiments in which depletion of CD8+ T-cells resulted in loss of the improved tumor control at TT. Additional analysis of several immune cell populations involved in the anti-tumor immune response revealed dramatic differences in mice at ST and TT. At TT, significantly greater numbers of CD8+ T-cells were present in 4T1 and Ct26 tumors (as assessed by both IHC and flow cytometry) and staining with pentamers recognizing the H-2Ld/gp70 peptide antigen of ct26 tumors, showed that increased numbers of antigen specific T-cells were found in both the tumor and tumor draining lymph node of mice housed at TT compared to ST. Correlating with their increased presence, T-cell activation was significantly higher at TT as judged by CD69, IFNγ and Glut-1 expression. Conversely, there were fewer immunosuppressive cells at TT; the numbers of Tregs (FoxP3+ cells) and myeloid derived suppressor cells (MDSC: CD11b+GR-1+) were significantly decreased in the tumor (T-regs) and spleen (MDSC) at TT. It is interesting that others have reported a trend to higher numbers of T-regs in tumors of mice (at ST) subjected to restraint/noise stress [104] . These differences in the anti-tumor immune response at TT vs ST are not the result of differences in body temperature since the core temperatures of these tumor-bearing mice maintained at 22°C or 30°C were normothermic for several weeks (~28 days). Only as tumor burden became significantly higher at ST than at TT did the core temperature fall in mice at ST, while mice at TT continued to maintain a normal temperature, reflecting the smaller tumor burden. In addition to CD8+T-cells and immune suppressor cells, in a separate study we also examined how housing temperature might impact antigen presenting cells; we investigated the function of dendritic cells (DCs), which are involved in T-cell activation. Results of these experiments suggest that DC’s from mice at TT (with 4T1 tumors) are better able to induce T-cell proliferation that are DC’s from mice at ST [107] suggesting another aspect of the anti-tumor immune response which is at least partially suppressed by housing mice at ST. Altogether, these findings point out that at ST, DC’s are less able to stimulate T-cells, and that the balance of anti-tumor (CD8+T-cells) and pro-tumor cells (T-regs, MDSC) is shifted to significantly suppress the anti-tumor immune response. Therefore, these data demonstrate that results from experiments conducted at ST are giving us a biased view of the activity and capabilities of the anti-tumor immune response. Thus, we strongly believe that temperature should always be considered and reported in experiments with an immune component and that investigators could gain important information by repeating selected experiments at TT rather than relying solely on the data from experiments conducted at ST only.
With regard to the direct tumor growth-promoting effects of adrenergic signaling on tumor cells, we have found that at ST (compared to TT) the level of NE is significantly higher in the plasma of non-tumor-bearing and in the plasma and tumors of pancreatic tumor-bearing mice [21]. It has previously been reported that the catecholamine levels are higher in the tissues of tumor-bearing mice subjected to restraint stress in experiments conducted at ST [101]. Interestingly, given the roles of epinephrine and corticosterone in certain types of stress, we found that the levels of these stress hormones are not significantly different at ST and TT. Because it has been reported that adrenergic signaling increases levels of anti-apoptotic molecules (phosphorylated BAD, [102]) and protects tumor cells from apoptosis [95, 102], we investigated the effect of ST vs. TT on apoptotic signaling and response to therapy [21]. We found that treatment of murine and human pancreatic adenocarcinoma cell lines in vitro with a β-AR agonist (isoproterenol) increased expression of anti-apoptotic molecules including Bcl-Xl, Bxl-2, Mcl-1 and phosphorylated BAD. The same differences in these anti-apoptotic molecules were seen in in vivo in tumors when these cell lines were grown in SCID mice housed at ST vs. TT. In SCID mice, as expected in the absence of the adaptive immune response, tumor growth at ST and TT was not significantly different. However, as suggested by the differences in expression of anti-apoptotic molecules, we found that tumors in mice housed at TT were significantly more sensitive to Apo2L/TRAIL, cisplatin and nab-paclitaxel (Abraxane) than tumors in mice at ST. Furthermore, tumors in mice at ST could be sensitized to these therapies by treating the mice with a β-adrenergic receptor antagonist (propranolol) which decreased the expression of these anti-apoptotic molecules [21]. These results show, for the first time, that the degree of stress experienced by mice housed at ST is sufficient to directly impact the outcome of experiments testing the efficacy of therapeutics and, for that reason, it is critical to also conduct these experiments at TT so that the results can be compared.
As these therapeutic studies indicated that giving β-blockers to mice housed at ST can overcome resistance to cytotoxic therapies and achieve responses comparable to those achieved at TT, we wondered whether propranolol could reverse immunosuppression at ST and similarly improve responses to immunotherapy. We recently have found that this is true[38]. Given to mice at ST, propranolol reverses immunosuppression increasing the frequency of CD8+T cells with an effector phenotype and increasing the CD8+ effector/ CD4+ T-reg ratio in the TME. The ability of propranolol to reduce suppressive cells in the TME and increase numbers of cytotoxic T-cells was also recently reported to occur in a spontaneous mouse melanoma model[108]. We have found that these changes in the immune contexture in the tumor (with either housing at TT or propranolol administration at ST) lead to significantly improved response to anti-PD-1 checkpoint inhibition [38]. These results support the development of clinical trials to explore using this combination strategy to benefit those patients who are not currently responding to checkpoint inhibitor therapies.
7. Mechanisms by which chronic adrenergic signaling suppresses the cellular immune response at ST
Immune cells express adrenergic receptors- primarily β2-AR, although they may express other receptors and the pattern is cell specific [56]. Anti-tumor immunity is primarily dependent on tumor cell killing by cytotoxic CD8+ lymphocytes (CTL), therefore boosting the efficacy of these cells against cancer is the focus of a spectrum of immunotherapies, for example, Chimeric Antigen Receptor T-cells are CD8+ T-cells taken from a cancer patient, engineered to express specific T-cell receptors (CAR-T-cells) which are chimeric in that they have intracellular domains that initiate T-cell activation. These cells are then expanded in vitro, and given back to the patient as adoptive T-cell therapy. Another exciting approach designed to improve T-cell anti-tumor activity is checkpoint inhibition. Checkpoint inhibitors work by modulating the activity of ligands/receptors (e.g. CTLA-4, PD-1/PD-L1) whose natural function is to keep the activity of these cells in check. Given the central, critical role of CTL (cytotoxic T lymphocytes, CD8+T cells) in the anti-tumor response and the growing efforts to maximize their efficacy, how does chronic adrenergic stress contribute to the suppression of these cells? As mentioned above, lymphoid organs are profusely innervated by sympathetic neurons, especially in T-cell areas [5], and Elenkov et al reported that stress hormones act on antigen presenting cells to promote a Th2 response (favoring B cells/plasma cell maturation and antibody production) to protect against extracellular pathogens [109, 110]. At the same time, in response to β2-AR stimulation, DC production of IL-12 is inhibited and this suppresses Th1 development which would support CTL development [111] while production of anti-inflammatory cytokines Il-10 and Il-6 is upregulated [110]. Another aspect of this skewing to a Th2 response is the fact that β2-AR receptors are expressed on Th1 CD4+ helper cells, but not on Th2 cells. Therefore, adrenergic signaling directly impacts cytokine production by Th1 cells (i.e. IL-12) but not by Th2 cells[112]. In experiments using a novel procedure for inducing stress in mice (exposure to stressful sound) bearing Ct26 tumors, there was a Th1 to Th2 shift as evidenced by levels of IFNγ and Il-4 and this correlated with increased tumor growth [113]. In addition to NE production and release by sympathetic post-ganglionic neurons, immune cells also can produce catecholamines; T-cells, macrophage and neutrophils can synthesize and secrete catecholamines that act in an autocrine and paracrine way to modulate an immune response [56]. Nguyen et al compared the production of catecholamines by adipose tissue associated macrophages at 4°C, 22 °C and 30°C and found that macrophage underwent alternative activation at the sub-thermoneutral temperatures. This was IL-4 (a Th2 cytokine) dependent and resulted in the increased production of both Epi and NE [114]. More recently, this idea has been challenged by Fischer et al who reported that alternatively activated macrophages do not produce NE[115]. It will be interesting however to determine whether tumor associated macrophages can produce NE and whether this contributes to higher intratumoral levels of NE at ST than at TT as this could be a second source of local NE production suppressing CTL in the tumor microenvironment. As discussed above, our group found that tumor-bearing mice had higher numbers of suppressor cells (Tregs and MDSC) at ST than at TT [24]. CD4+ T-regs express functional β2-ARs and adrenergic signaling increases cAMP and PKA dependent phosphorylation of the transcription factor CREB (cAMP response element binding protein) leading to increased suppressive function, including increased CTLA expression [116]. Jin et al [117] looked at the effects of restraint stress on MDSC accumulation in bone marrow and found that chronic stress significantly increased the number of MDSC (CD11B+Gr1+; predominantly Ly6C-Ly6G+) and that these were immature neutrophils. This skewing of myelopoiesis by chronic restraint stress could be reversed with propranolol (but not by inhibition of glucocorticoids). Altogether, these data underscore the detrimental effects of chronic adrenergic stress which overall suppresses effector T-cell responses while promoting the development and activities of immune suppressor cells. This potential for mild, housing induced cold stress to inhibit immune responses has been recently reviewed by our group [11].
8. How does adrenergic signaling affect patient outcomes?
Going forward, it is important to understand how these observations on the effect(s) of adrenergic stress induced in pre-clinical mouse models can be related to the clinic in terms of treating patients and improving therapeutic outcomes. Patients can be highly stressed by a wide range of stressors (e.g. physical such as pain and psychological such as fear and isolation). One highly relevant study found that ovarian cancer patients who lacked social support had higher levels of NE and epinephrine than patients and that overall this was associated with advanced stage and higher grade tumors [86]. How does this stress affect patient outcome? There are now a number of retrospective, epidemiological reports strongly supporting the idea that patients who are taking β-blockers for hypertension or another indication have better outcomes overall (see section 5 above). There are also retrospective reports that β-blockers can reduce the incidence of HCV-associated hepatocellular carcinoma [125]and improve responses to chemotherapy [120]. Thus the potential for these commonly prescribed and comparatively safe β-blockers to be repurposed to treat cancer patients is exciting, but the rationale must be validated in prospective, well-planned clinical trials.
Another way in which the pre-clinical data on cold-stress may have an impact relates to pre-clinical testing of therapies. It is possible, that under a range of conditions, some agents that appear ineffective in models may become effective (or show greater efficacy) when stress is reduced or blocked. These results could pave the way for combination therapies in clinical trials and/or allow lower doses to achieve efficacy thus reducing toxicity. It is also possible that toxicities that did not occur in pre-clinical studies and were therefore not predicted (e.g. autoimmunity with immunotherapies such as checkpoint inhibitors [126], could become apparent if experimental designs included stress reduction which reversed immunosuppression.
9. Other forms of stress impacting mice in research facilities
In light of the examples described above, it is a clear that we need to take the effect of stress into account when designing experiments in pre-clinical mouse models and interpreting the results. Our lab has focused on how housing temperature induced cold-stress skews experimental outcomes, but there are many other environmental variables that could also act as stress rheostats, increasing or decreasing the degree of adrenergic stress experienced by mouse disease models (see Fig 1). Because the outcomes of pre-clinical mouse studies form the basis for understanding tumor biology, host responses and determining which therapies to take into clinical trials [127–130]; it is critical that researchers are aware of these factors. One major problem that results from variability is irreproducibility [33, 127]. In two major studies by drug companies, Bayer [128]and Amgen [131]investigated the reproducibility of preclinical experiments and found that less than 25% and 11%, respectively, of the studies were able to be duplicated. Furthermore, a landmark study by Landis and colleagues was extremely critical of this lack of reproducibility and pointed to the general dearth of information on the “design, conduct and analysis of the experiments” [132]. These authors asserted that “a core set of research parameters must be defined and should be addressed when reporting the results of animal experiments” and stated that a “concerted effort by all stakeholders, including funding agencies and journals, will be necessary to disseminate and implement best reporting practices throughout the research community.”
Fig. 1:
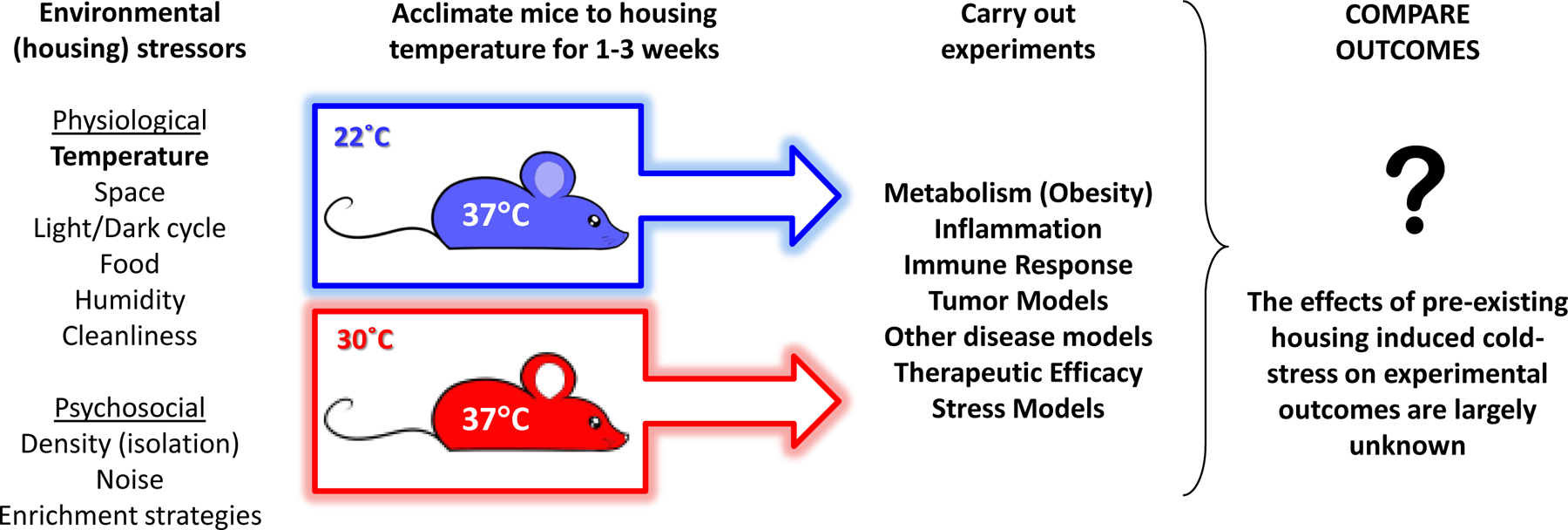
Housing guidelines for experimental mice regulate many environmental factors which affect the physiology of mice used in pre-clinical experiments; variations in these parameters can create differing degrees of stress. In the case of temperature, mice housed at standard sub-thermoneutral housing temperatures (22°C) are subjected to chronic cold-stress compared to mice housed at thermoneutral temperatures (30°C) and, although the body temperatures in both cases are normal, cold-stressed mice have elevated levels of norepinephrine. Thus these mice have a pre-existing level stress which is biologically significant and the effects of this stress on different experimental models is largely unknown.
For decades, institutions have adhered to The Guide for the Care and Use of Laboratory Animals (The Guide; [1]) which provides guidelines regulating all aspects of the research mouse environment (see 1 above). However, we are beginning to recognize the impact of these variables on the biology of mice and, recently, studies by others on non-tumor bearing mice (e.g. [6, 7, 133]) as well as our own research on cancer models [21, 24, 107], have convinced us that these housing choices have great potential to skew the outcome of experiments (see also Toth review [33]) . This viewpoint is echoed in a recent editorial by the editors of Nature Neurobiology who wrote: “Factors such as animal housing, handling, food, lighting and noise conditions, all of which effect behavior and brain chemistry, can be varied. The key to reproducibility is accurate reporting of these seemingly mundane details, which potentially have large effects” [134]. Demas and Carlton [135] have reviewed the potential for environmental factors to act on the nervous, immune and endocrine systems, affecting the biology of the mouse. Additionally, experimentally imposed psychosocial stresses such as repeated restraint [101, 104, 119]}, scream [113], variation in housing density [90]and social isolation [86, 136] have been shown to directly promote tumor cell proliferation, growth, survival and metastasis by increased adrenergic signaling (see recent review by [71]). Two recent studies have demonstrated the striking potential of environmentally induced stress to affect tumor growth. Li et al [137] and Garofalo et al [138]found that when mice were housed in an enriched environment which reduced stress/anxiety, the growth of pancreatic tumors and gliomas was significantly inhibited. These studies are also indicative of how stressful conditions of ST housing are since they show that reducing the stress experienced at ST improves tumor control. Garofalo et al [138]found that the improved tumor control can involve immune (innate) and non-immune mechanisms, however the role of β-adrenergic signaling was not addressed in these studies and this will be important to compare in the future. Clearly, housing factor induced psychosocial stress is a source of variability between experiments and labs. However, the degree to which environmental stresses caused by housing choices alters the levels of stress hormones and how this potentially impacts preclinical studies of cancer has received very little attention.
10. Conclusions
The tumor-promoting effects of chronic stress are currently the focus of research which provides a rationale for Clinical Trials to test whether β-blockers can be used in combination with chemotherapy and other therapies to improve patient outcome. In analyzing these pre-clinical data, what has not been appreciated is that housing conditions, particularly the sub-thermoneutral ambient temperatures, are subjecting these laboratory mice to a degree of chronic cold stress which is sufficient to raise NE levels, suppress the anti-tumor immune response and induce resistance to therapies tested using these models. Thus increased tumor growth arises from both an increase in the expression of anti-apoptotic molecules in the tumor cells themselves and suppression of the naturally occurring anti-tumor immune response. The implications of these observations are important in assessing how to design preclinical experiments that will maximize our understanding of diseases processes and how the immune response can be regulated to treat diseases, as well as obtaining a broad view of therapeutic responses. We predict that any therapy whose immediate or long-term outcome is even partially dependent on the anti-tumor immune response will be compromised in experiments conducted at ST. In fact, there are now several reports describing experiments whose outcomes are different when they are conducted at ST vs. TT [7, 12–14, 19–37]. We believe these studies serve as a caution against accepting the results from experiments conducted under one set of conditions as the “baseline” when in fact, the results may be significanlty different if parameters such as temperature are changed, as in our tumor growth experiments conducted as ST and TT. How can this housing cold-stress be overcome in traditional animal facilities? We have used incubators maintained at 22°C or 30°C ([21, 24, 25, 107] while others suggest using nesting materials in cages at ST [42, 43]. In any case, going forward, we believe that the housing temperatures and other environmental variables which can impact results, and are a likely source of experimental variability, should be reported in publications. Lastly, we encourage investigators conducting metabolic experiments, immunological investigations and therapeutic efficacy testing to consider comparing outcomes at both ST and TT.
Contributor Information
Bonnie L. Hylander, Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY, 14263
Jason W.-L. Eng, Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY
Elizabeth A. Repasky, Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY, 14263
References:
- 1.Animals NRCUCftUotGftCaUoL: Guide for the Care and Use of Laboratory Animals (National Academies, Washington, DC: ), 2011, 8th ed. [Google Scholar]
- 2.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS: Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341(6142):1236361. [DOI] [PubMed] [Google Scholar]
- 3.Szpunar MJ, Belcher EK, Dawes RP, Madden KS: Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain Behav Immun 2016, 53:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S: Noradrenergic and peptidergic innervation of lymphoid tissue. Journal of immunology (Baltimore, Md : 1950) 1985, 135(2 Suppl):755s–765s. [PubMed] [Google Scholar]
- 5.Nance DM, Sanders VM: Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 2007, 21(6):736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin B, Ji S, Maudsley S, Mattson MP: “Control” laboratory rodents are metabolically morbid: why it matters. Proceedings of the National Academy of Sciences of the United States of America 2010, 107(14):6127–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J: UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009, 9(2):203–209. [DOI] [PubMed] [Google Scholar]
- 8.Karp CL: Unstressing intemperate models: how cold stress undermines mouse modeling. The Journal of experimental medicine 2012, 209(6):1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodhi IJ, Semenkovich CF: Why we should put clothes on mice. Cell Metab 2009, 9(2):111–112. [DOI] [PubMed] [Google Scholar]
- 10.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM: Translating animal model research: does it matter that our rodents are cold? Physiology 2014, 29(6):413–420. [DOI] [PubMed] [Google Scholar]
- 11.Messmer MN, Kokolus KM, Eng JW, Abrams SI, Repasky EA: Mild cold-stress depresses immune responses: Implications for cancer models involving laboratory mice. BioEssays : news and reviews in molecular, cellular and developmental biology 2014, 36(9):884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overton J: Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes (Lond) 2010, 34(Suppl 2):S53–S58. [DOI] [PubMed] [Google Scholar]
- 13.Ravussin Y, LeDuc CA, Watanabe K, Leibel RL: Effects of ambient temperature on adaptive thermogenesis during maintenance of reduced body weight in mice. Am J Physiol Regul Integr Comp Physiol 2012, 303(4):R438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao C, Goldgof M, Gavrilova O, Reitman ML: Anti-obesity and metabolic efficacy of the beta3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22 degrees C. Obesity (Silver Spring) 2015, 23(7):1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David JM, Chatziioannou AF, Taschereau R, Wang H, Stout DB: The hidden cost of housing practices: using noninvasive imaging to quantify the metabolic demands of chronic cold stress of laboratory mice. Comparative medicine 2013, 63(5):386–391. [PMC free article] [PubMed] [Google Scholar]
- 16.Hylander BL, Repasky EA: Thermoneutrality, Mice and Cancer: A Heated Opinion. Trends in Cancer 2016, 2(4). [DOI] [PubMed] [Google Scholar]
- 17.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D: Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon CJ: Thermal physiology of laboratory mice: Defining thermoneutrality. Journal of Thermal Biology 2012, 37:654–685. [Google Scholar]
- 19.Cannon B, Nedergaard J: Brown adipose tissue: function and physiological significance. Physiol Rev 2004, 84:277–359. [DOI] [PubMed] [Google Scholar]
- 20.Cannon B, Nedergaard J: Thermogenesis challenges the adipostat hypothesis for bodyweight control. The Proceedings of the Nutrition Society 2009, 68(4):401–407. [DOI] [PubMed] [Google Scholar]
- 21.Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, Ma WW, Repasky EA, Hylander BL: Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta-adrenergic receptor activation. Nat Commun 2015, 6:6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golozoubova V, Gullberg H, Matthias A, Cannon B, Vennstrom B, Nedergaard J: Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Molecular endocrinology (Baltimore, Md) 2004, 18(2):384–401. [DOI] [PubMed] [Google Scholar]
- 23.Jhaveri KA, Trammell RA, Toth LA: Effect of environmental temperature on sleep, locomotor activity, core body temperature and immune responses of C57BL/6J mice. Brain Behav Immun 2007, 21(7):975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA: Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proceedings of the National Academy of Sciences of the United States of America 2013, 110(50):20176–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leigh ND, Kokolus KM, O’Neill RE, Du W, Eng JW, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X, Repasky EA: Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through beta2-Adrenergic Receptor Signaling. Journal of immunology (Baltimore, Md : 1950) 2015, 195(10):5045–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Povinelli BJ, Kokolus KM, Eng JW, Dougher CW, Curtin L, Capitano ML, Sailsbury-Ruf CT, Repasky EA, Nemeth MJ: Standard sub-thermoneutral caging temperature influences radiosensitivity of hematopoietic stem and progenitor cells. PloS one 2015, 10(3):e0120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA: Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 2005, 289(5):R1244–1252. [DOI] [PubMed] [Google Scholar]
- 28.Smith DL Jr., Yang Y, Hu HH, Zhai G, Nagy TR: Measurement of interscapular brown adipose tissue of mice in differentially housed temperatures by chemical-shift-encoded water-fat MRI. J Magn Reson Imaging 2013, 38(6):1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stemmer K, Kotzbeck P, Zani F, Bauer M, Neff C, Muller TD, Pfluger PT, Seeley RJ, Divanovic S: Thermoneutral housing is a critical factor for immune function and diet-induced obesity in C57BL/6 nude mice. Int J Obes (Lond) 2015, 39(5):791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM: Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 2008, 294(4):H1581–1588. [DOI] [PubMed] [Google Scholar]
- 31.Swoap SJ, Overton JM, Garber G: Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol 2004, 287(2):R391–396. [DOI] [PubMed] [Google Scholar]
- 32.Tian Xiao Y, Ganeshan K, Hong C, Nguyen Khoa D, Qiu Y, Kim J, Tangirala Rajendra K, Tonotonoz P, Chawla A: Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metabolism 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toth LA: The influence of the cage environment on rodent physiology and behavior: Implications for reproducibility of pre-clinical rodent research. Experimental neurology 2015. [DOI] [PubMed] [Google Scholar]
- 34.Uchida K, Shiuchi T, Inada H, Minokoshi Y, Tominaga M: Metabolic adaptation of mice in a cool environment. Pflugers Arch 2010, 459(5):765–774. [DOI] [PubMed] [Google Scholar]
- 35.Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N: Methodology of fever research: why are polyphasic fevers often thought to be biphasic? The American journal of physiology 1998, 275(1 Pt 2):R332–338. [DOI] [PubMed] [Google Scholar]
- 36.Hasday JD, Fairchild KD, Shanholtz C: The role of fever in the infected host. Microbes and infection / Institut Pasteur 2000, 2(15):1891–1904. [DOI] [PubMed] [Google Scholar]
- 37.Eng JW, Reed CB, Kokolus KM, Repasky EA: Housing temperature influences the pattern of heat shock protein induction in mice following mild whole body hyperthermia. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 2014, 30(8):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucsek M, Qiao G, MacDonald C, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW, Messmer MN, Atwood K, Abrams SI, Hylander BL, Repasky EA: β-adrenergic signaling in mouse models housed at standard temperatures suppresses an effector phenotype in CD8+T cells and undermines checkpoint inhibitor therapy 2017, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganeshan K, Chawla A: Warming the mouse to model human diseases. Nat Rev Endocrinol 2017, 13(8):458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrington LP: The heat regulation of small laboratory animals at various environmental temperatures. Amer J Physiol 1940, 129:123–139. [Google Scholar]
- 41.Gordon CJ: Relationship between autonomic and behavioral thermoregulation in the mouse. Physiology & behavior 1985, 34(5):687–690. [DOI] [PubMed] [Google Scholar]
- 42.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP: Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PloS one 2012, 7(3):e32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP: Impact of nesting material on mouse body temperature and physiology. Physiology & behavior 2013, 110–111:87–95. [DOI] [PubMed] [Google Scholar]
- 44.Gilon P, Henquin JC: Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocrine reviews 2001, 22(5):565–604. [DOI] [PubMed] [Google Scholar]
- 45.Teramura Y, Terao A, Okada Y, Tomida J, Okamatsu-Ogura Y, Kimura K: Organ-specific changes in norepinephrine turnover against various stress conditions in thermoneutral mice. The Japanese journal of veterinary research 2014, 62(3):117–127. [PubMed] [Google Scholar]
- 46.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP: Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387(6628):90–94. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP: Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. The Journal of clinical investigation 2003, 111(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anunciado-Koza R, Ukropec J, Koza RA, Kozak LP: Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. The Journal of biological chemistry 2008, 283(41):27688–27697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozak LP: Brown fat and the myth of diet-induced thermogenesis. Cell Metab 2010, 11(4):263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldgof M, Xiao C, Chanturiya T, Jou W, Gavrilova O, Reitman ML: The chemical uncoupler 2,4-dinitrophenol (DNP) protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. The Journal of biological chemistry 2014, 289(28):19341–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravussin Y: Temperature matters with rodent metabolic studies. Obesity (Silver Spring) 2015, 23(7):1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jun JC, Shin MK, Yao Q, Devera R, Fonti-Bevans S, Polotsky VY: Thermoneutrality modifies the impact of hypoxia on lipid metabolism. American journal of physiology Endocrinology and metabolism 2013, 304(4):E424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koizumi A, Wada Y, Tuskada M, Kayo T, Naruse M, Horiuchi K, Mogi T, Yoshioka M, Sasaki M, Miyamaura Y, Abe T, Ohtomo K, Walford R: A tumor preventive effect of dietary restriciton is antagonized by a high housing temperature throught deprivation of torpor. Mechanisms of Ageing and Development 1996, 92:67–82. [DOI] [PubMed] [Google Scholar]
- 54.Glaser R, Kiecolt-Glaser JK: Stress-induced immune dysfunction: implications for health. Nature reviews Immunology 2005, 5(3):243–251. [DOI] [PubMed] [Google Scholar]
- 55.Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R: Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Annals of the New York Academy of Sciences 1998, 840:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marino F, Cosentino M: Adrenergic modulation of immune cells: an update. Amino acids 2013, 45(1):55–71. [DOI] [PubMed] [Google Scholar]
- 57.Dhabhar FS: Effects of stress on immune function: the good, the bad, and the beautiful. Immunologic research 2014, 58(2–3):193–210. [DOI] [PubMed] [Google Scholar]
- 58.Moragues V, Pinkerton H: Variation in morbidity and mortality of murine typhus infection in mice with changes in the environmental temperature. The Journal of experimental medicine 1944, 79(1):41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Underwood GE, Baker CA, Weed SD: Protective effect of elevated temperature on mice infected with Coe virus. Journal of immunology (Baltimore, Md : 1950) 1966, 96(6):1006–1012. [PubMed] [Google Scholar]
- 60.Wang JF, Meissner A, Malek S, Chen Y, Ke Q, Zhang J, Chu V, Hampton TG, Crumpacker CS, Abelmann WH, Amende I, Morgan JP: Propranolol ameliorates and epinephrine exacerbates progression of acute and chronic viral myocarditis. Am J Physiol Heart Circ Physiol 2005, 289(4):H1577–1583. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Miss Mdel R, Mut-Martin MC, Gongora-Alfaro JL: beta-Adrenergic blockade protects BALB/c mice against infection with a small inoculum of Leishmania mexicana mexicana (LV4). International immunopharmacology 2015, 24(1):59–67. [DOI] [PubMed] [Google Scholar]
- 62.Montazeri M, Daryani A, Ebrahimzadeh M, Ahmadpour E, Sharif M, Sarvi S: Effect of Propranolol Alone and in Combination with Pyrimethamine on Acute Murine Toxoplasmosis. Jundishapur journal of microbiology 2015, 8(9):e22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grebe KM, Hickman HD, Irvine KR, Takeda K, Bennink JR, Yewdell JW: Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proceedings of the National Academy of Sciences of the United States of America 2009, 106(13):5300–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell JF, Moore GJ: Effects of high ambient temperature on various stages of rabies virus infection in mice. Infection and immunity 1974, 10(3):510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Repasky EA, Evans SS, Dewhirst MW: Temperature matters! And why it should matter to tumor immunologists. Cancer immunology research 2013, 1(4):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sen A, Capitano ML, Spernyak JA, Schueckler JT, Thomas S, Singh AK, Evans SS, Hylander BL, Repasky EA: Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer research 2011, 71(11):3872–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dewhirst MW, Lee CT, Ashcraft KA: The future of biology in driving the field of hyperthermia. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 2016, 32(1):4–13. [DOI] [PubMed] [Google Scholar]
- 68.Evans SS, Repasky EA, Fisher DT: Fever and the thermal regulation of immunity: the immune system feels the heat. Nature reviews Immunology 2015, 15(6):335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Padgett DA, Glaser R: How stress influences the immune response. Trends in Immunology 2003, 24(8):444–448. [DOI] [PubMed] [Google Scholar]
- 70.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK: Sympathetic nervous system regulation of the tumour microenvironment. Nature reviews Cancer 2015, 15(9):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cole SW, Sood AK: Molecular pathways: beta-adrenergic signaling in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2012, 18(5):1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA: A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer immunology, immunotherapy : CII 2014, 63(11):1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melhem-Bertrandt A, Sood AK: Adrenergic signaling and cancer: deciphering the connections. Cancer Biomark 2013, 13(3):131–132. [DOI] [PubMed] [Google Scholar]
- 74.Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, Entschladen F: Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1(7):628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K: Beta blockers and breast cancer mortality: a population- based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011, 29(19):2635–2644. [DOI] [PubMed] [Google Scholar]
- 76.Diaz ES, Karlan BY, Li AJ: Impact of beta blockers on epithelial ovarian cancer survival. Gynecologic oncology 2012, 127(2):375–378. [DOI] [PubMed] [Google Scholar]
- 77.Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, Matsuo K, Squires KC, Coleman RL, Lutgendorf SK, Ramirez PT, Sood AK: Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer 2015, 121(19):3444–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N, Geppetti P: Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 2011, 171(8):779–781. [DOI] [PubMed] [Google Scholar]
- 79.Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, Zhuang Y, Levy LB, Lu C, Gomez DR: Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2013, 24(5):1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grytli HH, Fagerland MW, Fossa SD, Tasken KA: Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. European urology 2014, 65(3):635–641. [DOI] [PubMed] [Google Scholar]
- 81.Udumyan R, Montgomery S, Fang F, Almroth H, Valdimarsdottir U, Ekbom A, Smedby KE, Fall K: Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer research 2017. [DOI] [PubMed] [Google Scholar]
- 82.Kim SA, Moon H, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY: Postdiagnostic use of beta-blockers and other antihypertensive drugs and the risk of recurrence and mortality in head and neck cancer patients: an observational study of 10,414 person-years of follow-up. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2017. [DOI] [PubMed] [Google Scholar]
- 83.Cardwell CR, Coleman HG, Murray LJ, Entschladen F, Powe DG: Beta-blocker usage and breast cancer survival: a nested case-control study within a UK Clinical Practice Research Datalink cohort. International journal of epidemiology 2013, 42(6):1852–1861. [DOI] [PubMed] [Google Scholar]
- 84.Cardwell CR, Coleman HG, Murray LJ, O’Sullivan JM, Powe DG: Beta-blocker usage and prostate cancer survival: A nested case-control study in the UK Clinical Practice Research Datalink cohort. Cancer epidemiology 2014. [DOI] [PubMed] [Google Scholar]
- 85.Hicks BM, Murray LJ, Powe DG, Hughes CM, Cardwell CR: beta-Blocker usage and colorectal cancer mortality: a nested case-control study in the UK Clinical Practice Research Datalink cohort. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2013, 24(12):3100–3106. [DOI] [PubMed] [Google Scholar]
- 86.Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, Goodheart M, Buekers TE, Mendez L, Krueger G, Clevenger L, Lubaroff DM, Sood AK, Cole SW: Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun 2011, 25(2):250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Masur K, Niggemann B, Zanker KS, Entschladen F: Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer research 2001, 61(7):2866–2869. [PubMed] [Google Scholar]
- 88.Palm D, Lang K, Niggemann B, Drell TLt, Masur K, Zaenker KS, Entschladen F: The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. International journal of cancer Journal international du cancer 2006, 118(11):2744–2749. [DOI] [PubMed] [Google Scholar]
- 89.Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, Renne G, Gandini S, Pouton CW, Ferrari D, Moller A, Stacker SA, Sloan EK: Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun 2016, 7:10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hasegawa H, Saiki I: Psychosocial stress augments tumor development through beta-adrenergic activation in mice. Japanese journal of cancer research : Gann 2002, 93(7):729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Wadei HA, Plummer HK 3rd, Ullah MF, Unger B, Brody JR, Schuller HM: Social stress promotes and gamma-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancer. Cancer prevention research (Philadelphia, Pa) 2012, 5(2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin Q, Wang F, Yang R, Zheng X, Gao H, Zhang P: Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PloS one 2013, 8(4):e61435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW: Stress hormone-mediated invasion of ovarian cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research 2006, 12(2):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Creed SJ, Le CP, Hassan M, Pon CK, Albold S, Chan KT, Berginski ME, Huang Z, Bear JE, Lane JR, Halls ML, Ferrari D, Nowell CJ, Sloan EK: beta2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast cancer research : BCR 2015, 17(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, Carroll AR, Spannuth WA, Deavers MT, Allen JK, Han LY, Kamat AA, Shahzad MM, McIntyre BW, Diaz-Montero CM, Jennings NB, Lin YG, Merritt WM, DeGeest K, Vivas-Mejia PE, Lopez-Berestein G, Schaller MD, Cole SW, Lutgendorf SK: Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. The Journal of clinical investigation 2010, 120(5):1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, Cole SW: The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer research 2010, 70(18):7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagaraja AS, Dorniak PL, Sadaoui NC, Kang Y, Lin T, Armaiz-Pena G, Wu SY, Rupaimoole R, Allen JK, Gharpure KM, Pradeep S, Zand B, Previs RA, Hansen JM, Ivan C, Rodriguez-Aguayo C, Yang P, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK: Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene 2016, 35(18):2390–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park SY, Kang JH, Jeong KJ, Lee J, Han JW, Choi WS, Kim YK, Kang J, Park CG, Lee HY: Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. International journal of cancer Journal international du cancer 2011, 128(10):2306–2316. [DOI] [PubMed] [Google Scholar]
- 99.Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, Barsky SH, Glaser R: Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun 2009, 23(2):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R: Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer research 2006, 66(21):10357–10364. [DOI] [PubMed] [Google Scholar]
- 101.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK: Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine 2006, 12(8):939–944. [DOI] [PubMed] [Google Scholar]
- 102.Sastry KS, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A, Carson JP, Weber MJ, Register TC, Chen YQ, Penn RB, Kulik G: Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. The Journal of biological chemistry 2007, 282(19):14094–14100. [DOI] [PubMed] [Google Scholar]
- 103.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D’Agostino R Jr., Danial N, Datta SR, Kulik G: Behavioral stress accelerates prostate cancer development in mice. The Journal of clinical investigation 2013, 123(2):874–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Partecke LI, Speerforck S, Kading A, Seubert F, Kuhn S, Lorenz E, Schwandke S, Sendler M, Kessler W, Trung DN, Oswald S, Weiss FU, Mayerle J, Henkel C, Menges P, Beyer K, Lerch MM, Heidecke CD, von Bernstorff W: Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology : official journal of the International Association of Pancreatology 2016, 16(3):423–433. [DOI] [PubMed] [Google Scholar]
- 105.He D, Manzoni A, Florentin D, Fisher W, Ding Y, Lee M, Ayala G: Biologic effect of neurogenesis in pancreatic cancer. Human pathology 2016, 52:182–189. [DOI] [PubMed] [Google Scholar]
- 106.Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C, Montero MP, Serdjebi C, Kavallaris M, Andre N: Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget 2011, 2(10):797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kokolus KM, Spangler HM, Povinelli BJ, Farren MR, Lee KP, Repasky EA: Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naive and tumor-bearing mice. Frontiers in immunology 2014, 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wrobel LJ, Bod L, Lengagne R, Kato M, Prevost-Blondel A, Le Gal FA: Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elenkov IJ, Chrousos GP: Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends in endocrinology and metabolism: TEM 1999, 10(9):359–368. [DOI] [PubMed] [Google Scholar]
- 110.Elenkov IJ, Chrousos GP: Stress system--organization, physiology and immunoregulation. Neuroimmunomodulation 2006, 13(5–6):257–267. [DOI] [PubMed] [Google Scholar]
- 111.Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F: Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. The Journal of clinical investigation 1997, 100(6):1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE: Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. Journal of immunology (Baltimore, Md : 1950) 1997, 158(9):4200–4210. [PubMed] [Google Scholar]
- 113.Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, Huang C: A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochemical and biophysical research communications 2013, 439(4):471–476. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A: Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480(7375):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, Kalinovich AV, Petrovic N, Wolf Y, Clemmensen C, Shin AC, Divanovic S, Brombacher F, Glasmacher E, Keipert S, Jastroch M, Nagler J, Schramm KW, Medrikova D, Collden G, Woods SC, Herzig S, Homann D, Jung S, Nedergaard J, Cannon B, Tschop MH, Muller TD, Buettner C: Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nature medicine 2017, 23(5):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guereschi MG, Araujo LP, Maricato JT, Takenaka MC, Nascimento VM, Vivanco BC, Reis VO, Keller AC, Brum PC, Basso AS: Beta2-adrenergic receptor signaling in CD4+ Foxp3+regulatory T cells enhances their suppressive function in a PKA-dependent manner. European journal of immunology 2013, 43(4):1001–1012. [DOI] [PubMed] [Google Scholar]
- 117.Jin J, Wang X, Wang Q, Guo X, Cao J, Zhang X, Zhu T, Zhang D, Wang W, Wang J, Shen B, Gao X, Shi Y, Zhang J: Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PloS one 2013, 8(9):e74497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM: Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011, 29(19):2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin CS, Lin WS, Lin CL, Kao CH: Carvedilol use is associated with reduced cancer risk: A nationwide population-based cohort study. International journal of cardiology 2015, 184:9–13. [DOI] [PubMed] [Google Scholar]
- 120.Giampieri R, Scartozzi M, Del Prete M, Faloppi L, Bianconi M, Ridolfi F, Cascinu S: Prognostic Value for Incidental Antihypertensive Therapy With beta-Blockers in Metastatic Colorectal Cancer. Medicine 2015, 94(24):e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, Lesinski GB, Jackson R, Glaser R: beta-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2011, 20(10):2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choi CH, Song T, Kim TH, Choi JK, Park JY, Yoon A, Lee YY, Kim TJ, Bae DS, Lee JW, Kim BG: Meta-analysis of the effects of beta blocker on survival time in cancer patients. Journal of cancer research and clinical oncology 2014, 140(7):1179–1188. [DOI] [PubMed] [Google Scholar]
- 123.Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I: Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proceedings of the National Academy of Sciences of the United States of America 2004, 101(21):8186–8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McCourt C, Coleman HG, Murray LJ, Cantwell MM, Dolan O, Powe DG, Cardwell CR: Beta-blocker usage after malignant melanoma diagnosis and survival: a population-based nested case-control study. The British journal of dermatology 2014, 170(4):930–938. [DOI] [PubMed] [Google Scholar]
- 125.Nkontchou G, Aout M, Mahmoudi A, Roulot D, Bourcier V, Grando-Lemaire V, Ganne-Carrie N, Trinchet JC, Vicaut E, Beaugrand M: Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer prevention research (Philadelphia, Pa) 2012, 5(8):1007–1014. [DOI] [PubMed] [Google Scholar]
- 126.Liu J, Blake SJ, Smyth MJ, Teng MW: Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin Transl Immunology 2014, 3(8):e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Freedman L, Gibson M: The impact of preclinical irreproducibility on drug development. Clinical pharmacology and therapeutics 2015, 97(1):16–18. [DOI] [PubMed] [Google Scholar]
- 128.Prinz F, Schlange T, Asadullah K: Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 2011, 10(9):712. [DOI] [PubMed] [Google Scholar]
- 129.Schein PS, Scheffler B: Barriers to efficient development of cancer therapeutics. Clinical cancer research : an official journal of the American Association for Cancer Research 2006, 12(11 Pt 1):3243–3248. [DOI] [PubMed] [Google Scholar]
- 130.Talmadge JE, Singh RK, Fidler IJ, Raz A: Murine models to evaluate novel and conventional therapeutic strategies for cancer. The American journal of pathology 2007, 170(3):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Begley CG, Ellis LM: Drug development: Raise standards for preclinical cancer research. Nature 2012, 483(7391):531–533. [DOI] [PubMed] [Google Scholar]
- 132.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD: A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012, 490(7419):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Macri S, Ceci C, Altabella L, Canese R, Laviola G: The Directive 2010/63/EU on animal experimentation may skew the conclusions of pharmacological and behavioural studies. Scientific reports 2013, 3:2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Editors: Troublesome variability in mouse studies. Nature neuroscience 2009, 12(9):1075. [DOI] [PubMed] [Google Scholar]
- 135.Demas GE, Carlton ED: Ecoimmunology for psychoneuroimmunologists: Considering context in neuroendocrine-immune-behavior interactions. Brain Behav Immun 2015, 44:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Madden KS, Szpunar MJ, Brown EB: Early impact of social isolation and breast tumor progression in mice. Brain Behav Immun 2013, 30 Suppl:S135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li G, Gan Y, Fan Y, Wu Y, Lin H, Song Y, Cai X, Yu X, Pan W, Yao M, Gu J, Tu H: Enriched Environment Inhibits Mouse Pancreatic Cancer Growth and Down-regulates the Expression of Mitochondria-related Genes in Cancer Cells. Scientific reports 2015, 5:7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garofalo S, D’Alessandro G, Chece G, Brau F, Maggi L, Rosa A, Porzia A, Mainiero F, Esposito V, Lauro C, Benigni G, Bernardini G, Santoni A, Limatola C: Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat Commun 2015, 6:6623. [DOI] [PMC free article] [PubMed] [Google Scholar]


