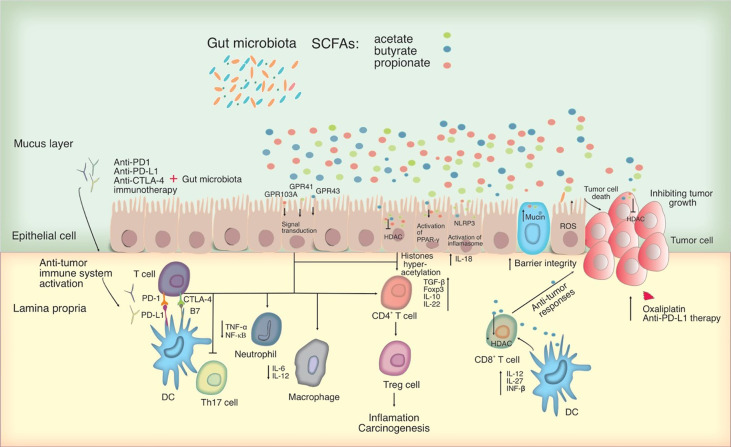
Figure 2.
Potential mechanisms for microbiota-mediated immunomodulation in tumor cells. SCFAs, which primarily consist of acetate, butyrate, and propionate can act as an HDAC inhibitor and influencing directly on cancer cells. By interacting with particular GPCRs including GPR41, GPR43 and GPR109A, SCFAs can have an effect on the immune system, leading to upregulation of immunosuppressive IL-10 and transforming growth factor-beta (TGF-β), downregulation of pro-inflammatory cytokines in macrophages and neutrophils, and inhibition of differentiation towards T helper type 17 (Th17) cells, thereby suppressing inflammation and carcinogenesis. SCFAs activate the inflammasome and the PPAR-γ pathway, promoting mucin production and improving epithelial integrity. SCFAs were also shown to activate the NLR family pyrin domain containing 3 (NLRP3) inflammasome, modulating the production of IL-18, which protects epithelial integrity. Significantly, SCFAs, in particular butyrate, may alter CD8+ T cell antitumor responses by influencing DC signaling pathways involving IL-12, IL-27, and IFN- β, all of which have an impact on tumor combination therapy. In this figure, the role of PD-1 and CTLA-4 in the priming and effector phases of anti-tumor immune responses is shown. Anti-CTLA-4 blocking antibodies may thereby restore T cell priming in lymph nodes, whereas PD-1 signaling inhibition may allow T cells to operate as tumor effectors. Other cell types in the tumor microenvironment, such as DCs, may also express PD-1 and hence be impacted by PD-1 inhibition. Blocking PD-1 and CTLA-4 may influence T helper cell profiles directly or indirectly by changing the microbiota. HDAC, Histone deacetylase; PD-L, Programmed death-ligand; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; PPAR-γ, peroxisome proliferator-activated receptor-γ; GPCRs, G-protein-coupled receptors.