Abstract
Background
During the 2020 COVID-19 lockdown, patients included in the Interprofessional Medication Adherence Program (IMAP) in Switzerland continued to use electronic monitors (EMs) that registered daily drug-dose intake. We aimed to understand to what extent patients’ medication implementation (ie, the extent to which the patient took the prescribed medicine), measured with EMs, was impacted by the lockdown.
Methods
Patients participating in the IMAP were diagnosed with diabetic kidney disease (DKD), solid cancer, human immunodeficiency virus (HIV) and miscellaneous long-term diseases (MLTD). Patient implementation was defined through a proxy: if all patient EMs were opened at least once daily, implementation was considered active (=1), and no implementation was considered (=0) otherwise. Implementation before (from December 2019 to March 2020), during (March to June 2020) and after (June to September 2020) the lockdown was compared. Subanalyses were performed according to the patients’ diseases. Subanalyses were performed in patients who used at least one EM in 2018–2019 during the same periods (defined as winter, spring and summer). The logistic regression models used to estimate medication implementation according to the period were fitted using generalized estimating equations.
Results
In 2020, patient implementation (n = 118) did not differ significantly before versus during (OR = 0.98, 95% CI: 0.84–1.15, p = 0.789) and before versus after (OR = 0.91, 95% CI: 0.79–1.06, p = 0.217) the lockdown. These findings remained stable when separately analyzing the implementation of patients with HIV (n = 61), DKD (n = 25) or MLTD (n = 22). Too few patients with cancer were included (n = 10) to interpret the results. In 2019, the implementation of 61/118 (51.7%) patients was significantly lower during summertime versus wintertime (OR = 0.73, 95% CI: 0.60–0.89, p = 0.002).
Conclusion
Medication implementation remained steady before, during and after the lockdown in 2020. The IMAP before, during and after the lockdown may have supported the adherence of most patients, by ensuring continuity of care during periods of routine disturbances.
Keywords: SARS-CoV-2, COVID-19, medication adherence, patient compliance, implementation adherence, electronic adherence monitoring, interprofessional adherence intervention
Introduction
Medication nonadherence in patients with chronic diseases is a major economic and public health concern.1 Medication adherence is the extent to which a patient manages a prescription, whether the medication is prescribed according to shared decision-making or not. It is characterized by initiation (whether the patient takes the first dose), implementation (daily treatment intake) and discontinuation (prematurely stopping the treatment).2 Persistence describes the time between initiation and discontinuation. Adherence can be affected by more than 700 determinants,3 including social support, access to medication,4 communication with health care providers (HCPs), and socioeconomic or employment status, which were impacted by the lockdown due to the Coronavirus disease (COVID)-19 pandemic.
Indeed, during the COVID-19 lockdown in Switzerland, from March to June 2020, the Swiss authorities strongly recommended that the population stay at home: working from home was mandatory; schools, social and cultural places were closed; social gatherings were prohibited, including in nursing homes; only essential shops were accessible; and Swiss frontiers were closed for nonworkers.
Literature about patients’ medication adherence during this period is scarce and often measured by retrospective self-reports.5–7 Adherence during lockdown varied according to the monitored treatment, the disease and the patient’s perceived risk: in a German study, 84% of patients with inflammatory rheumatic diseases treated with immunomodulatory therapies reported that they continued the treatment, and only 3% discontinued treatment. Patients’ behaviour towards medication management remained stable before, during and after the lockdown.8 The adherence of patients with asthma and chronic obstructive pulmonary disease in the USA, evaluated through electronic monitors (EM) during the first 7 days of January 2020 and compared to the last 7 days of March 2020, increased from 54% to 62%.9 In India, patients who missed their medical appointments in a glaucoma clinic during the lockdown answered a questionnaire by phone call regarding their medication adherence. In total, 57% were considered non-adherent. Barriers to adherence were medicine supply issues, financial difficulties and no perceived effectiveness of the antiglaucoma medications.10 To our knowledge, no longitudinal adherence data are available on patients’ adherence in Switzerland during the lockdown.
The aim of the CovADH (Covid and ADHerence to medication) study is to analyze EM data of patients included in the Interprofessional Medication Adherence Program (IMAP)11 to describe patients’ medication implementation before, during and after the 2020 lockdown. We hypothesized that medication implementation was impacted during and after the lockdown compared to before the lockdown.
Methods
The CovADH study is a retrospective analysis that combines the anonymous databases of several prospective studies and cohorts from the IMAP. The local ethics committee (Vaud, Switzerland) authorized the CovADH study to be run after the anonymization of the databases. All included patients signed either the IMAP-specific informed consent form or the Swiss HIV (Human Immunodeficiency Virus) cohort study (SHCS)-specific informed consent form, which allows researchers to use patients’ adherence data for research purposes.
The IMAP runs routinely at the outpatient community pharmacy of the Center for Primary Care and Public Health (Unisanté, Lausanne, Switzerland)11 and at the Department of Infectious Diseases of the University Hospital in Bern (Inselspital, Bern, Switzerland). Patients included are mostly adults, able to manage their treatment on their own (ie, do not receive home care services) and without any major cognitive impairment. The IMAP consists of repeated one-on-one motivational interviews between a patient and an HCP to support medication self-management and adherence.12,13 Patients included in IMAP use at least one EM (MEMS and MEMS AS; AARDEX Group, Sion, Switzerland), each of which contains a patient’s medication. EMs register the date and hour of each opening in real time as a proxy of drug intake. During the lockdown, the IMAP organization was adapted to avoid patients coming to the pharmacy (eg, medications were mailed, interventions were delivered by phone or in person to patients known to have adherence issues).14
The following IMAP participants were included in the CovADH database: patients with diabetic kidney disease (DKD) from the PANDIA-IRIS study,15 patients with solid cancer from the OpTAT study,16 participants of the SHCS and patients with miscellaneous long-term diseases (MLTD) (eg, hypertension, diabetes, heart diseases, renal failure). To be eligible for inclusion, participants had to use one or more EMs during the lockdown (the minimum monitored period was 47 days).
As the combined database is anonymous, patient daily regimens and the expected number of daily EM openings were not known. Thus, patients’ implementation was defined through a proxy: if all EMs used by a patient were opened at least once daily, medication implementation was considered as active (=1) and no implementation was considered (=0) otherwise. EM data with an oral chemotherapy cyclic regimen were excluded from the analysis. Hence, implementation was expressed as the number of days where a patient opened each EM at least once divided by the number of monitored days during the monitored period.
Patient implementation was compared among three periods: before (December 1, 2019 to March 15, 2020), during (March 16 to June 7, 2020) and after (June 8 to September 30, 2020) the lockdown. The implementation of subgroups of patients diagnosed with solid cancer, HIV, DKD or MLTD was compared.
Implementation of the same patients who also used one or more EMs during Spring 2019 (the minimum monitored period was 74 days) during the same time frame, defined as winter (December 1, 2018 to March 15, 2019), spring (March 16 to June 7, 2019) and summer (June 8 to September 30, 2019), served to compare variation within a year.
To obtain a clean database, all consecutive EM nonopenings equal to or more than 6 consecutive days (arbitrary choice of length established upon database observations) were set as nonmonitored periods to prevent misinterpretations of EM nonopenings (eg, hospitalization, prescribed treatment interruptions, treatment use without an EM). In the case of initiation of EM use after the 1st of December or stopping EM use before September 30 of the following year, the label “missing data” was imputed between December 1st and EM initiation or from EM stopping until September 30, respectively. This EM data cleaning process prepared the database, named the “clean EM database”, for implementation analysis in both 2019 and 2020.
In total, analyses were performed on three different databases, including 2019 and 2020 EM data: the main analysis was processed with the clean EM database, and two sensitivity analyses were performed to confirm its validity. The first sensitivity analysis, called “A”, was performed on the “Raw database” (ie, the unclean database without any nonmonitored periods). The second sensitivity analysis, called “B”, was performed on the “Restricted database”, a clean database including only the EM data of patients who used their EM during the entire period of interest (ie, from December 1 to September 30 of the following year).
A logistic regression model was used to estimate the implementation according to the period, using “before the lockdown” in the 2020 analysis (“winter” in the 2019 analysis) as the reference. The models were fitted using Generalized Estimating Equations (GEE) because of the unknown correlation between the repeated observations of the patients (each patient was observed multiple times, ie, once per day). The level of statistical significance was set at p < 0.05. EM database cleaning and statistical analysis were performed using R statistical software.17
Results
Of the 118 patients (10 patients with cancer, 25 with DKD, 61 with HIV and 22 with MLTD) included in the 2020 implementation analysis, 61 (51.7%) (1 patient with cancer, 6 with DKD, 41 with HIV, 13 with MLTD) were also included in the 2019 implementation analysis.
Sensitivity analysis A (ie, using the raw database) included the same number of patients. Sensitivity analysis B (ie, including only patients who used EMs during the entire period) included 60/118 (50.8%) patients from the 2020 clean database and 31/61 (50.8%) patients from the 2019 clean database.
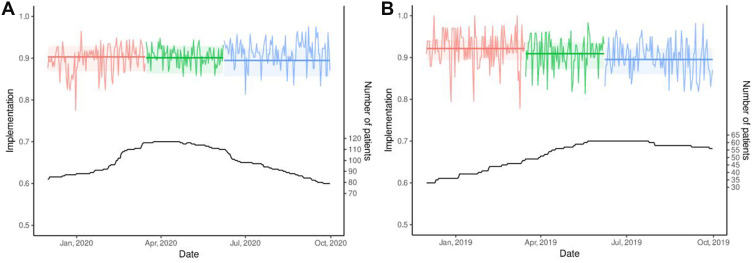
In 2020, the probability of active implementation was 90.3% before, 90.1% during and 89.5% after the lockdown. Patient implementation did not differ before versus during (OR = 0.98, 95% CI: 0.84–1.15, p = 0.789) and before versus after (OR = 0.91, 95% CI: 0.79–1.06, p = 0.217) the lockdown (Table 1). Figure 1 depicts the empirical implementation and the GEE modelisations within each period, respectively, in 2020 (a) and 2019 (b) using the clean database. The results of sensitivity analyses A (Supplementary Materials 1 and 2) and B (Supplementary Materials 3 and 4) were similar.
Table 1.
Patient Medication Implementation in 2020 and 2019 Using the Clean Database
| EM Database 2020 (n = 118) | ||||||||
|---|---|---|---|---|---|---|---|---|
| All Patients (n = 118) | ||||||||
| Periods | Implementation | Odds Ratio | ||||||
| Estimate | 95% CI | Estimate | 95% CI | p-value | ||||
| Beforea | 0.903 | 0.869 | 0.929 | NA | ||||
| Duringb | 0.901 | 0.863 | 0.929 | 0.979 | 0.835 | 1.147 | 0.789 | |
| Afterc | 0.895 | 0.856 | 0.923 | 0.911 | 0.787 | 1.056 | 0.217 | |
| Patients with cancer (n = 10) | ||||||||
| Beforea | 0.964 | 0.889 | 0.989 | NA | ||||
| Duringb | 0.962 | 0.891 | 0.987 | 0.945 | 0.755 | 1.182 | 0.619 | |
| Afterc | 0.942 | 0.821 | 0.983 | 0.610 | 0.512 | 0.726 | <0.001g | |
| Patients with diabetic kidney disease (n = 25) | ||||||||
| Beforea | 0.972 | 0.948 | 0.985 | NA | ||||
| Duringb | 0.962 | 0.931 | 0.979 | 0.731 | 0.485 | 1.100 | 0.133 | |
| Afterc | 0.960 | 0.932 | 0.977 | 0.701 | 0.462 | 1.063 | 0.094 | |
| Patients with HIV (n = 61) | ||||||||
| Beforea | 0.892 | 0.842 | 0.928 | NA | ||||
| Duringb | 0.905 | 0.853 | 0.939 | 1.146 | 0.959 | 1.368 | 0.133 | |
| Afterc | 0.893 | 0.840 | 0.930 | 1.008 | 0.822 | 1.237 | 0.938 | |
| Miscellaneous patients (n=22) | ||||||||
| Beforea | 0.861 | 0.765 | 0.922 | |||||
| Duringb | 0.828 | 0.706 | 0.907 | 0.781 | 0.534 | 1.140 | 0.200 | |
| Afterc | 0.835 | 0.713 | 0.911 | 0.818 | 0.633 | 1.056 | 0.124 | |
| EM database 2019 (n = 61) | ||||||||
| All patients (n = 61) | ||||||||
| Periods | Implementation | Odds Ratio | ||||||
| Estimate | 95% CI | Estimate | 95% CI | p-value | ||||
| Winterd | 0.922 | 0.894 | 0.942 | NA | ||||
| Springe | 0.909 | 0.873 | 0.936 | 0.852 | 0.706 | 1.029 | 0.097 | |
| Summerf | 0.895 | 0.860 | 0.923 | 0.728 | 0.596 | 0.891 | 0.002 | |
Notes: aFrom December 1, 2019 to March 15, 2020; bFrom March 16 to June 7, 2020; cFrom June 8 to September 30, 2020; dFrom December 1, 2018 to March 15, 2019; eFrom March 16 to June 7, 2019; fFrom June 8 to September 30, 2019; gThis result is not interpretable as only 5/10 (50%) patients with cancer were still using the EM on September 30, 2020.
Abbreviation: NA, not applicable (reference period).
Figure 1.
Empirical implementation and GEE modelisations in 2020 (A) and 2019 (B) using the clean database. (A) Period  Before
Before  During
During  After. (B) Period
After. (B) Period  Winter
Winter Spring
Spring  Summer.
Summer.
In 2019, the probability for active implementation was 92.2% in the winter, 90.9% in the spring and 89.5% in the summer. Patient implementation was significantly lower during summertime than during wintertime (OR = 0.73, 95% CI: 0.60–0.89, p = 0.002) (Table 1, Figure 1). Again, the results were similar in sensitivity analysis A (Supplementary Materials 1 and 2). In sensitivity analysis B, implementation was significantly lower in the summer and spring compared with the winter (Supplementary Materials 3 and 4). In 2020, the probability for active implementation of the 61 patients who used their EM both in 2019 and 2020 was stable across the periods: implementation was 87.6% before, 86.6% during and 86.2% after the 2020 lockdown (Supplementary Materials 5 and 6).
Subgroups of Patients in 2020
The implementation of patients with HIV, DKD or other MLTD did not differ significantly among the periods in 2020 (there were too few patients with cancer at the end of the monitoring period to allow for analysis) (Table 1). Both sensitivity analyses showed some changes in implementation among periods according to the disease: for instance in sensitivity analysis A, patients with HIV implemented their treatment better during the lockdown (+3%), and the implementation of patients with MLTD decreased during (−10%) and after (−6%) the lockdown compared to before the lockdown (Supplementary Material 1).
Discussion
Our results do not support our hypothesis, as medication implementation remained steady before, during and after the lockdown in 2020, and the results were similar across patient subgroups.
On the one hand, implementation may have been positively impacted by the lockdown, eg, many patients slowed down their activities, traveled less, and were more cautious in managing their chronic treatment due to the fear of developing disease complications in a difficult sanitary context. On the other hand, implementation may also have been negatively impacted by social isolation and the loss of workday routines. We hypothesize that both tendencies could have counterbalanced each other. Moreover, during the pandemic, continuity of care was ensured by medical teleconsultations and phone calls between patients and their HCPs. Pharmacists mailed medications to patients’ homes and led interviews by phone calls with patients included in the IMAP. Despite fewer medical and pharmacist face-to-face consultations, adherence was not impaired.
When analyzing subgroups of patients, implementation was quite similar across groups except for the group of patients diagnosed with MLTD, whose implementation rate was lower. These patients came from different medical backgrounds, and probably had less threatening diseases than patients in the other subgroups. The sample size was relatively small, and caution must be taken when interpreting the results.
In 2019, medication adherence decreased during the summertime, but this phenomenon did not occur after the lockdown in the summer 2020; this is potentially linked to the fact that the population had just emerged from the lockdown with much uncertainty about the evolution of the pandemic.
The main strengths of this study rely on the longitudinal, electronic monitoring of medication implementation before, during and after the lockdown and on the sensitivity analyses, which strengthen the results.
The main limitation of this study is the evaluation of medication implementation through a proxy. This proxy, which considered all regimens as QD (once-daily) regimens, might have overestimated adherence in patients who were prescribed a more frequent regimen, such as a BID (twice-daily) regimen. In contrast, adherence might have been underestimated if implementation was low for one drug but adequate for others. Nevertheless, the same proxy was used to evaluate implementation across the various periods (before, during and after the lockdown), which allowed us to analyze the change in implementation between periods rather than the implementation rate itself. Second, some patients were prescribed cotreatments that were not monitored by EMs, and their implementation could have differed from those that were monitored. However, the monitored medications were among the most relevant medications that our participants were taking. Third, although only half (61/118; 52%) of the participants in 2020 were taking part in the IMAP in 2019, the sample remained quite substantial to provide a comparative overview of the change of implementation across periods in 2019. Finally, the timing of drug intake could have been shifted during the lockdown due to the dismantling of routines, but due to ethical requirements, we were not allowed to investigate this dimension, as unusual timing could enable the identification of some patients. Also, because of ethical requirements linked to the anonymization of the database, we were not allowed to provide sample characteristics (eg, age, gender, education). Further studies should investigate the sociodemographic determinants of adherence during the 2020 COVID-19 lockdown.
Conclusions
Medication implementation remained steady before, during and after the lockdown in 2020. According to our results, it is unlikely that medication adherence was negatively impacted by the lockdown. The IMAP may have contributed to supporting the medication adherence of patients throughout the pandemic. Interprofessional adherence programs that are tailored to patients’ needs, ensure continuity of care and avoid gaps in medication supply by the regular mailing of treatment contribute to supporting patients during periods of routine disturbances such as lockdowns in a pandemic context.
Acknowledgments
The authors would like to thank all the collaborators from the IMAP at the outpatients community pharmacy of the Center for Primary Care and Public Health Unisanté (Lausanne, Switzerland) and of the Inselspital (Bern, Switzerland).
Funding Statement
The Optat study is funded by the Swiss Cancer Research Foundation (grant HSR-4077-11-2016) and the PANDIA-IRIS study by LOA IV fund, managed by curafutura, pharmaSuisse and santésuisse (Switzerland). The funding bodies did not intervene in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Abbreviations
BID, Twice-daily regimen; CovADH, Covid and ADHerence to medication study; COVID, COronaVIrus Disease; DKD, Diabetic Kidney disease; EM, Electronic Monitor; GEE, Generalized Estimating Equations; HCPs, Health Care Providers; HIV, Human Immunodeficiency Virus; IMAP, Interprofessional Medication Adherence Program; MLTD, Miscellaneous Long-Term Diseases; QD, Once-daily regimen; SHCS, Swiss HIV Cohort Study; USA, United States of America.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The local ethics committee (Comité d’éthique de la Recherche du canton de Vaud, Switzerland) authorized the CovADH study to be run after anonymization of the databases. All included patients signed an IMAP or SHCS-specific informed consent form, which allows researchers to use EM data of patients for research purposes. The study was performed in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
Prof. Dr. Grégoire Wuerzner reports consultancy and/or advisory board from Aktiia, Bayer, Servier and AstraZeneca, outside the submitted work. Prof. Dr. Gilles Wandeler reports grants, Speaker and advisory board fees paid to their institution from Gilead Sciences and MSD, grants from Roche Diagnostics, advisory board fees paid to their institution from ViiV, outside the submitted work. Dr Anna Dorothea Wagner reports personal fees, non-financial support from Merck, personal fees from Lily, personal fees from Pierre-Fabre Pharma, is coordinating investigator of EORTC trial 1203 “INNOVATION”, which is supported by an educational grant to EORTC from Hoffmann La Roche, personal fees from Sanofi, personal fees from Daiichi Sankyo, personal fees from Dragonfly Therapeutics, personal fees from Servier, personal fees from Bristol-Myers Squibb, personal fees from Astellas Pharma, outside the submitted work.
Professor Chantal Csajka reports grants from Swiss Cancer Research, during the conduct of the study. Prof. Dr. Matthias Cavassini reports grants from Gilead, ViiV and MSD, outside the submitted work.
Professor Marie Schneider reports grants from Swiss Cancer Research Foundation, grants from Research and quality fund of Santésuisse, Curafutura and PharmaSuisse, during the conduct of the study. The authors declare that they have no other competing interests.
References
- 1.Khan R, Socha-Dietrich K. Investing in Medication Adherence Improves Health Outcomes and Health System Efficiency. Paris: OECD Publishing; 2018. [Google Scholar]
- 2.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91. doi: 10.3389/fphar.2013.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pineda-Sic RA, Galarza-Delgado DA, Serna-Peña G, et al. Treatment adherence behaviours in rheumatic diseases during COVID-19 pandemic: a Latin American experience. Ann Rheum Dis. 2020;80(6):e85. [DOI] [PubMed] [Google Scholar]
- 5.Zhang HQ, Lin JY, Guo Y, Pang S, Jiang R, Cheng QJ. Medication adherence among patients with chronic obstructive pulmonary disease treated in a primary general hospital during the COVID-19 pandemic. Ann Transl Med. 2020;8(18):1179. doi: 10.21037/atm-20-6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorfman L, Nassar R, Binjamin Ohana D, et al. Pediatric inflammatory bowel disease and the effect of COVID-19 pandemic on treatment adherence and patients’ behavior. Pediatr Res. 2021;90(3):637–641. doi: 10.1038/s41390-020-01312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Midao L, Almada M, Carrilho J, Sampaio R, Costa E. Pharmacological adherence behavior changes during COVID-19 outbreak in a Portugal patient cohort. Int J Environ Res Public Health. 2022;19(3):1135. doi: 10.3390/ijerph19031135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasseli R, Muller-Ladner U, Keil F, et al. The influence of the SARS-CoV-2 lockdown on patients with inflammatory rheumatic diseases on their adherence to immunomodulatory medication - a cross sectional study over 3 months in Germany. Rheumatology. 2021;60(SI):SI51–SI58. doi: 10.1093/rheumatology/keab230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaye L, Theye B, Smeenk I, Gondalia R, Barrett MA, Stempel DA. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(7):2384–2385. doi: 10.1016/j.jaip.2020.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subathra GN, Rajendrababu SR, Senthilkumar VA, Mani I, Udayakumar B. Impact of COVID-19 on follow-up and medication adherence in patients with glaucoma in a tertiary eye care centre in south India. Indian J Ophthalmol. 2021;69(5):1264–1270. doi: 10.4103/ijo.IJO_164_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lelubre M, Kamal S, Genre N, et al. Interdisciplinary medication adherence program: the example of a university community pharmacy in Switzerland. Biomed Res Int. 2015;2015:103546. doi: 10.1155/2015/103546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lelubre M, Clerc O, Grosjean M, et al. Implementation study of an interprofessional medication adherence program for HIV patients in Switzerland: quantitative and qualitative implementation results. BMC Health Serv Res. 2018;18(1):874. doi: 10.1186/s12913-018-3641-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–473. doi: 10.1037/0278-6133.25.4.462 [DOI] [PubMed] [Google Scholar]
- 14.Bourdin A, Dotta-Celio J, Niquille A, Berger J. Response to the first wave of the COVID-19 pandemic in the community pharmacy of a university center for primary care and public health. Res Social Adm Pharm. 2022;18(4):2706–2710. doi: 10.1016/j.sapharm.2021.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandiera C, Dotta-Celio J, Locatelli I, et al. Interprofessional medication adherence program for patients with diabetic kidney disease: protocol for a randomized controlled and qualitative study (PANDIA-IRIS). JMIR Res Protoc. 2021;10(3):e25966. doi: 10.2196/25966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandiera C, Cardoso E, Locatelli I, et al. Optimizing oral targeted anticancer therapies study for patients with solid cancer: protocol for a randomized controlled medication adherence program along with systematic collection and modeling of pharmacokinetic and pharmacodynamic data. JMIR Res Protoc. 2021;10(6):e30090. doi: 10.2196/30090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R-development-core-team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. Available from: http://www.R-project.org. [Google Scholar]