Abstract
The APOE locus is strongly associated with risk for developing Alzheimer’s disease and dementia with Lewy bodies. In particular, the role of the APOE ε4 allele as a putative driver of α-synuclein pathology is a topic of intense debate.
Here, we performed a comprehensive evaluation in 2466 dementia with Lewy bodies cases versus 2928 neurologically healthy, aged controls. Using an APOE-stratified genome-wide association study approach, we found that GBA is associated with risk for dementia with Lewy bodies in patients without APOE ε4 (P = 5.65 × 10−8, OR = 3.21, 95% CI = 2.11–4.88), but not with dementia with Lewy bodies with APOE ε4 (P = 0.034, OR = 1.87, 95%, 95% CI = 1.05–3.37). We then divided 495 neuropathologically examined dementia with Lewy bodies cases into three groups based on the extent of concomitant Alzheimer’s disease co-pathology: pure dementia with Lewy bodies (n = 88), dementia with Lewy bodies with intermediate Alzheimer’s disease co-pathology (n = 66) and dementia with Lewy bodies with high Alzheimer’s disease co-pathology (n = 341). In each group, we tested the association of the APOE ε4 against the 2928 neurologically healthy controls.
Our examination found that APOE ε4 was associated with dementia with Lewy bodies + Alzheimer’s disease (P = 1.29 × 10−32, OR = 4.25, 95% CI = 3.35–5.39) and dementia with Lewy bodies + intermediate Alzheimer’s disease (P = 0.0011, OR = 2.31, 95% CI = 1.40–3.83), but not with pure dementia with Lewy bodies (P = 0.31, OR = 0.75, 95% CI = 0.43–1.30).
In conclusion, although deep clinical data were not available for these samples, our findings do not support the notion that APOE ε4 is an independent driver of α-synuclein pathology in pure dementia with Lewy bodies, but rather implicate GBA as the main risk gene for the pure dementia with Lewy bodies subgroup.
Keywords: dementia with Lewy bodies, APOE, Alzheimer’s disease, co-pathology
Is APOE ε4 also a driver of α-synuclein pathology? Kaivola et al. show that APOE ε4 is associated with dementia with Lewy bodies only in those patients with Alzheimer’s disease co-pathology. The absence of an association between APOE ε4 and pure dementia with Lewy bodies supports the existence of distinct disease subtypes.
Introduction
Dementia with Lewy bodies (DLB) is a fatal neurological disease characterized by variable combinations of fluctuating cognition, parkinsonism, visual hallucinations and rapid eye movement behaviour disorder.1 This form of dementia is among the most common neurological diseases in the general population, accounting for ∼7.5% of all dementia cases.2 There are currently no effective disease-modifying treatments available and the prognosis is poor. Because of the significant morbidity associated with this understudied disease, the healthcare costs associated with DLB are among the highest for any age-related disease.3
Clinical, neuropathological and genomic studies have shown that DLB exists along a continuum involving Alzheimer’s disease and Parkinson’s disease. The core neuropathological features of DLB are Lewy bodies and Lewy neurites composed primarily of abnormally phosphorylated α-synuclein deposits.1 These pathological hallmarks are also present in Parkinson’s disease, although they are typically not as widespread. The majority of DLB patients show Alzheimer’s disease co-pathology consisting of amyloid-β plaques and neurofibrillary tangles.4 Our recent genome-wide association study (GWAS) in Lewy body dementia identified five genome-wide significant risk loci: GBA, BIN1, TMEM175, SNCA and APOE.5 Of these, GBA, SNCA and TMEM175 are well-established Parkinson’s disease risk loci that are crucial in the production and regulation of α-synuclein.6-8 At the same time, APOE and BIN1 are known Alzheimer’s disease risk loci that affect the accumulation of both amyloid-β and neurofibrillary tangles.9,10
Despite these advances, the interplay between Alzheimer’s disease, Parkinson’s disease and DLB is complex and poorly understood. In particular, the role of the APOE ε4 allele as a possible independent driver of α-synuclein pathology in DLB remains a topic of intense debate. Two recent studies in human α-synuclein transgenic mice expressing different human APOE isoforms found that the APOE ε4 allele regulates synucleinopathies directly and independently of amyloid-β deposition.11,12 Post-mortem human studies also reported that APOE ε4 is associated with DLB regardless of the severity of concomitant Alzheimer’s disease pathology.12–14 In contrast, other studies found that APOE ε4 is only associated with disease when there is considerable Alzheimer’s disease co-pathology.15,16 Notably, a recent population-based study showed that Lewy body pathology progresses in two distinct patterns and Alzheimer’s disease co-pathology and APOE ε4 are only associated with one of them.17 If true, this finding implicates the existence of multiple distinct DLB subtypes. Such disease heterogeneity may explain the disparate results discovered by previous studies.
Here, we explored the role of APOE ε4 in the pathogenesis of DLB. To do this, we investigated whether APOE ε4 is associated with risk for developing DLB regardless of the presence or absence of Alzheimer’s disease co-pathology. These analyses are based on a sizable whole-genome sequencing data set generated from patients diagnosed with DLB, providing adequate power to resolve this critical aspect of the neurological disease.5
Materials and methods
Sample cohorts and genome sequencing
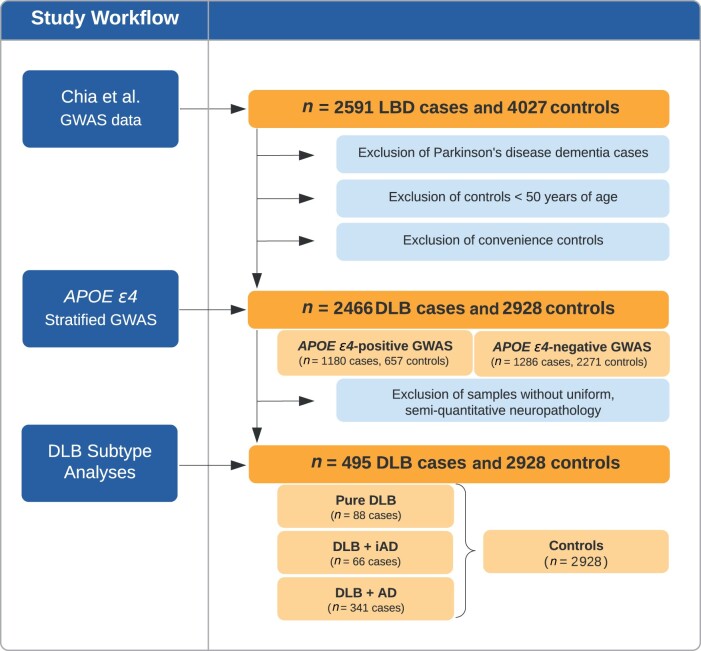
Fig. 1 shows the analysis pipeline used in this study. We used genomic data from our recently published Lewy body dementia GWAS based on 2592 Lewy body dementia cases and 4027 neurologically healthy control subjects.5 All study participants were of European descent and were diagnosed based on consensus criteria1,18 or were neurologically healthy individuals as described elsewhere.5 Whole-genome sequencing was performed on an Illumina HiSeq X Ten platform using 150-bp paired-end cycles. Alignment (using the GRCh38DH reference genome) and variant calling followed the GATK Best Practices.19 Sample-level and variant-level quality control steps have been described elsewhere.5 This study was approved by the appropriate institutional review boards of the participating institutions. All participants or their surrogate decision makers gave informed consent according to the Declaration of Helsinki.
Figure 1.
Analysis overview. This schematic illustration of the study workflow shows the cohort selection and analysis steps. AD = Alzheimer’s disease; LBD = Lewy body dementia; iAD = intermediate-level Alzheimer’s disease co-pathology.
The APOE-stratified GWASs were performed using samples selected from the overall cohort of 2466 DLB cases and 2928 neurologically healthy controls. Patients diagnosed with Parkinson’s disease dementia, controls under the age of 50 years and convenience controls where the neurological status was unclear were excluded from the selection process. The pathology subtype analysis was restricted to the 495 patients who were (i) pathologically diagnosed as DLB using the McKeith criteria1; and (ii) for whom uniformly collected semiquantitative Alzheimer’s disease co-pathology measures were available.
Neuropathological subgrouping
The 495 definite DLB cases were categorized into three subgroups based on the severity of the Alzheimer’s disease co-pathology. The extent of amyloid-β pathology was quantified using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scoring20 and neurofibrillary tangle pathology was staged using the Braak method.21 The three subgroups were: (i) pure DLB, defined as absent or low Alzheimer’s disease co-pathology (Braak stages 0–2 and CERAD scores 0–A); (ii) DLB with intermediate Alzheimer’s disease co-pathology (corresponding to Braak stage 3 and CERAD scores A–C); and (iii) DLB with high Alzheimer’s disease co-pathology (Braak stages 4–6 and CERAD scores B–C).
Genetic analysis
The ε4 APOE allele was identified based on the genotypes at two common single nucleotide polymorphisms (rs7412 and rs429358). We assessed the association of the APOE ε4 allele (presence or absence) with DLB by performing two GWASs. In the first GWAS, we evaluated the DLB cases without any APOE ε4 allele and compared them to neurologically healthy controls without APOE ε4. In the second GWAS, we compared the DLB cases with at least one APOE ε4 allele to healthy controls who were carrying at least one APOE ε4 allele.
In addition to the APOE ε4-stratified GWASs, we tested the associations of the APOE ε4 allele with each of the three pathologically defined subgroups (pure DLB, DLB + intermediate Alzheimer’s disease and DLB + Alzheimer’s disease) versus all of the controls. We also tested the associations of the rs2230288 GBA risk allele with each of the three pathological subgroups versus controls.
Statistical analyses
APOE ε4-stratified analyses
GWAS testing and association analysis were performed in PLINK (version 2.0) using an additive model with a minor allele frequency threshold of 1%.22 Age, sex and relevant principal components to account for population stratification were included as covariates. The top ten principal components were calculated using FlashPCA. We determined the significant principal components to include in each analysis using the ‘step’ function (Ripley), as incorporated in the R (version 3.5.2, https://www.R-project.org) ‘stats’ package. The principal components included in these analyses were as follows: (i) principal component 1, 2, 3 and 4 in the APOE ε4-negative DLB cases versus controls GWAS; and (ii) 1, 2 and 10 in the APOE ε4-positive DLB cases versus controls GWAS. The threshold for genome-wide significance was 5.0 × 10−8.
Subgroup analysis
We performed the APOE ε4 analysis in DLB subgroups using the ‘glm’ function under a dominant association model, as implemented in the R stats package.23 The principal components included in the subtype analyses were as follows: (i) 1, 2 and 6 in the APOE and GBA allele analysis in the pure DLB cohort versus controls; (ii) 1, 4, 5, 6, 7 and 10 in the APOE and GBA allele analysis in the DLB + intermediate Alzheimer’s disease cohort versus controls; and (iii) 1, 2, 3, 4, 5, 6 and 7 in the APOE and GBA allele analysis in the DLB + Alzheimer’s disease cohort versus controls. Association results for Bonferroni-corrected for multiple testing using a P-value threshold of 0.017 (= 0.05/3 groups tested).
Data availability
Individual-level sequence data are available on dbGaP (accession number: phs001963.v1.p1). The analysis presented here has not been previously published elsewhere.
Results
APOE ε4-stratified GWAS
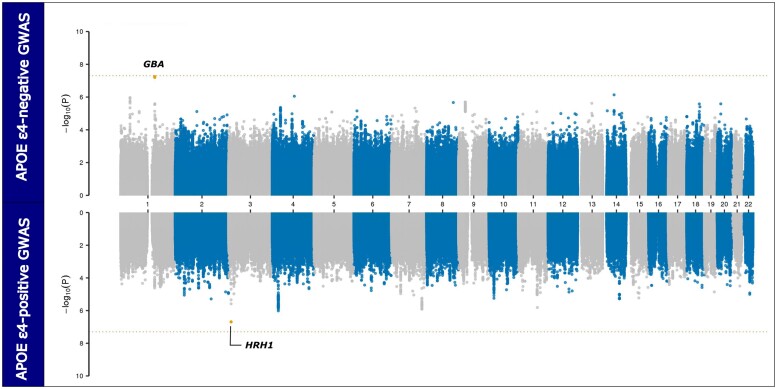
We explored the genetic risk factors among DLB patients carrying and not carrying the APOE ε4 allele. To perform this stratified GWAS, we compared the 1286 DLB cases without APOE ε4 to the 2271 controls without APOE ε4. The genomic inflation factor λ1000 was 1.009, indicative of only minimal residual population stratification. GBA was the only locus that nearly reached genome-wide significance in this analysis (rs2230288, P = 6.58 × 10−9, OR = 3.41, 95% CI = 2.25–5.17; Fig. 2). When we compared the 1180 DLB cases with APOE ε4 to the 657 controls with APOE ε4, the GBA locus signal did not achieve genome-wide significance (P = 0.034, OR = 1.87, 95% CI = 1.05–3.37), suggesting that GBA is not a major determinant of disease risk in APOE ε4 carriers. However, we noted a subsignificant association signal within the histamine receptor H1 (HRH1) gene (rs9858388, P = 2.0 × 10−7, OR = 1.47, 95% CI = 1.27–1.71). Furthermore, no association signals exceeded the Bonferroni threshold for multiple testing in the APOE ε4-positive GWAS. The λ1000 for this GWAS was 1.012. These findings confirmed the importance of GBA as a significant driver of α-synuclein pathology in the APOE e4-negative DLB patients.
Figure 2.
Miami plot depicting the APOE-stratified GWAS results. The upper panel shows the GWAS results comparing APOE ε4-negative DLB cases with APOE ε4-negative controls (n = 1286 cases versus 2271 controls). The bottom panel shows the association test results comparing APOE ε4-positive DLB cases with APOE ε4-positive controls (n = 1180 cases versus 657 controls). The x-axis depicts the chromosomal position for 22 autosomes in hg38 and the y-axis denotes the association P-values on a −log10 scale. The dotted, horizontal line indicates the conservative Bonferroni threshold for genome-wide significance. Suggestive variants are indicated by orange dots, while red dots highlight genome-wide significant associations.
APOE associations with DLB subgroups
Of the 495 DLB cases with available co-pathology measures, 88 (17.8%) were classified as pure DLB cases, 66 (13.3%) cases were categorized as having intermediate AD co-pathology and 341 (68.9%) were identified as having severe AD co-pathology. Table 1 shows the clinical and demographic details of these subgroups. Men were overrepresented in the pure DLB group (81%). Only limited phenotype data were available for these individuals.
Table 1.
DLB subgroups and demographic characteristics
| Pure DLB | DLB + intermediate Alzheimer’s disease | DLB + Alzheimer’s disease | Controls | |
|---|---|---|---|---|
| n | 88 | 66 | 341 | 2928 |
| Mean age (SD) | 73 (11) | 79 (10) | 76 (11) | 78 (11) |
| Age range, years | 40–95 | 55–100 | 39–103 | 50–110 |
| % Male | 81 | 59 | 52 | 46 |
| APOE ε4 carriers | ||||
| Homozygous (%) | 0 (0%) | 2 (3%) | 47 (14%) | 42 (1%) |
| Heterozygous (%) | 17 (19%) | 25 (38%) | 148 (43%) | 615 (21%) |
| GBA rs2230288T carriers (%)a | 7 (8%) | 3 (5%) | 9 (3%) | 51 (2%) |
One pure DLB case was homozygous for the rs2230288T risk allele, while all other GBA risk allele carriers were heterozygous.
APOE ε4 was strongly associated with disease in the DLB with severe Alzheimer’s disease co-pathology subgroup (P = 1.29 × 10−32, OR = 4.25, 95% CI = 3.35–5.39) and the DLB with intermediate AD co-pathology subgroup (P = 0.0011, OR = 2.31, 95% CI = 1.40–3.83). In contrast, APOE ε4 was not associated with disease in the pure DLB cohort (P = 0.31, OR = 0.75, 95% CI = 0.43–1.30). Moreover, DLB patients with high Alzheimer’s disease co-pathology were more likely to be homozygous for the APOE ε4 allele than the other subgroups displaying less severe Alzheimer’s disease co-pathology [n = 47 (13.8%) in the DLB + Alzheimer’s disease group, n = 2 (3.0%) in the DLB + intermediate Alzheimer’s disease group and n = 0 (0.0%) in the pure DLB group; Fisher P-value = 4.4 × 10−6], consistent with dose-dependent effects on disease risk. Taken together, these findings do not support a role of APOE ε4 as an independent driver of human α-synuclein pathology.
In contrast to the APOE ε4 subgroup associations, we found a statistically significant association of the GBA rs2230288 risk allele with the pure DLB subgroup (P = 0.0004, OR = 4.52, 95% CI = 1.94–10.44). Interestingly, we did not identify an association within the intermediate or high Alzheimer’s disease co-pathology subgroups (DLB + intermediate Alzheimer’s disease: P = 0.11, OR = 2.67, 95% CI = 0.80–8.89; DLB + Alzheimer’s disease: P = 0.32, OR = 1.45, 95% CI = 0.69–3.01). These findings support the existence of distinct genetic architectures within each DLB subtype.
Discussion
The influence of genetic association signals implicated in Lewy body dementia on Alzheimer’s disease co-pathology has been unclear. APOE ε4 is the most common genetic risk factor for late-onset Alzheimer’s disease, and it has also been consistently the top association signal for Lewy body dementia.5,14,24,25 Controversial evidence exists implicating APOE ε4 as an independent driver of α-synuclein pathology. Here, we show that the association of APOE ε4 with DLB is dependent on the severity of Alzheimer’s disease co-pathology, as APOE ε4 was associated with DLB only when there were intermediate or high levels of Alzheimer’s disease co-pathology. No associations were found for APOE ε4 with pure DLB, arguing against the notion that APOE ε4 is an independent driver of α-synuclein pathology.
We made several additional observations. First, in the APOE-stratified GWAS, we found that the GBA risk variant rs2230288 nearly reached genome-wide significance when comparing DLB cases without APOE ε4 to healthy controls without APOE ε4. In contrast, we did not detect any genome-wide significant loci when examining DLB cases with APOE ε4. Taken together, these findings demonstrate a clear relationship between GBA and APOE ε4-negative DLB, whereas the association with APOE ε4-positive DLB is equivocal. However, we noticed a subsignificant signal within the HRH1 gene, encoding the histamine receptor H1 that is widely expressed within the central nervous system. Histaminergic dysregulation is a crucial feature of Alzheimer’s disease and DLB,26,27 making HRH1 a plausible risk gene. However, additional genetic association studies will be required to determine the importance of this observation. Furthermore, the rs2230288 variant located within the GBA locus was associated with pure DLB (P-value = 0.0004, OR = 4.52, 95% CI = 1.95–10.44) but not with DLB with Alzheimer’s disease co-pathology (P-value = 0.32, OR = 1.45, 95% CI = 0.69–3.01). Overall, these findings suggest the existence of DLB subgroups with distinct genetic architectures, perhaps hallmarked by the APOE and GBA loci.
Only a limited number of DLB research studies have previously accounted for the severity of Alzheimer’s disease co-pathology. While some studies reported the association of APOE with DLB to be dependent on the presence of Alzheimer’s disease co-pathology,15,16 others did not.12–14 One possible explanation for this discrepancy in the literature may be the small sample sizes and varying neuropathological definitions for pure DLB. In addition, each study employed different inclusion and exclusion criteria and methodologies to group the neuropathological changes. For example, in one of the previous studies, the aged controls had to be free of cognitive impairment both at study enrolment and at the last evaluation. Such criteria may have led to a selection bias against APOE ε4, and the results may be attributed to the lack of APOE ε4 in cognitively intact aged individuals rather than its association with DLB. Other co-pathologies, such as microvascular disease and TDP-43 inclusions, could be present in this aged cohort and may explain the disparate results in the studies. Such co-pathologies were more likely to have emerged if the patients had survived longer. These data were not available for the samples that were included in our analysis.
The relationship of APOE to other genetic and non-genetic risk factors is complex. For example, transgenic mouse models expressing the human APOE ε4 allele and a pathogenic mutation in SNCA, encoding the α-synuclein protein, showed increased α-synuclein aggregation.12 However, it is difficult to extrapolate from artificial model systems to human patients. Additional factors, such as aging, sex, polygenic genetic contributions of small effect size, cerebrovascular disease, mitochondrial impairment, neuroinflammation and dysfunctional lysosomes may interact with APOE, and the outcome likely depends on the integrated sum of these factors.28 Our study highlights the value of studying neurological diseases directly in pathology-derived human tissue as a means to understand the primary drivers underlying co-pathologies.
Aside from genetic differences, we observed that 81% of the pure DLB group were male, compared to the DLB + Alzheimer’s disease group, where the male-to-female ratio was ∼1. This observation is in line with previous studies of DLB with varying severity of Alzheimer’s disease co-pathology.13,14,16 Because all studies, including ours, have potential selection biases and confounding factors that affect sex, we cannot conclude that sex influences the DLB phenotype. However, the consistency with which males form the majority of pure DLB cases is noteworthy. Interestingly, the male sex has also been implicated as a risk factor for Parkinson’s disease with the same neuropathological changes as pure DLB.29
A strength of our study is the availability of neuropathological data from a large cohort of patients diagnosed with DLB. These data allowed for a careful exploration of the genetic effects on co-pathology. Despite this, the absolute number of our patient collection was relatively small compared to the larger-scale GWASs that are standard in the field today. Although interesting, our results must be confirmed in more extensive studies that longitudinally collect clinical, cognitive and neuropathological information, such as quantifications of TDP-43 co-pathology and microangiopathic changes. Analysis of such clinical information would provide additional insights into the genetic factors driving cognitive decline across DLB subtypes and across males and females. More extensive studies are also required to determine the relative importance of common variation and rare mutations in GBA, a locus where the risk is known to be pleomorphic.5 Another limitation of our study is that all participants were individuals of European ancestry. It will be essential to include diverse populations in future efforts to obtain a comprehensive understanding of the genetic drivers underlying DLB.
In conclusion, our data show that APOE ε4 is not an independent driver of α-synuclein pathology in DLB. Instead, the severity of Alzheimer’s disease co-pathology influences the association of APOE ε4. Based on this, it is clear that the severity of Alzheimer’s disease co-pathology should be considered in future genetic studies, as missing neuropathological subgroups may obscure association signals. Moreover, considering the severity of Alzheimer’s disease co-pathology may make it easier to determine the manner in which α-synuclein and Alzheimer’s disease pathology interact in DLB. The severity of Alzheimer’s disease co-pathology, and the corresponding underlying genetics, may be used to assign patients to subgroups, each with different symptoms and each requiring specific targeted treatments.
Supplementary Material
Acknowledgements
We thank the patients and families whose help and participation made this work possible. We thank the members of the International LBD Genomics Consortium (a complete list of site investigators who contributed samples for genome sequencing can be found in the Appendix). We thank Dr Bryan Traynor and the Laboratory of Neurogenetics (NIH) for their collegial support and technical assistance. This work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Funding
This study was supported in part by the Intramural Research Program of the National Institutes of Health (National Institute on Aging, National Institute of Neurological Disorders and Stroke; project numbers: 1ZIAAG000935, 1ZIANS003154). K.K. was funded by the Finnish Cultural Foundation, The Finnish Parkinson Foundation, The Päivikki and Sakari Sohlberg Foundation and The Finnish Brain Foundation.
Competing interests
S.W.S. serves on the Scientific Advisory Council of the Lewy Body Dementia Association. S.W.S. is an editorial board member for the Journal of Parkinson’s Disease and JAMA Neurology. All other authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary material
Supplementary material is available at Brain online.
Abbreviations
- DLB
dementia with Lewy bodies
- GWAS
genome-wide association study
Appendix I
International LBD Genomics Consortium
Full details are provided in the Supplementary material.
Sandra E. Black, Ziv Gan-Or, Julia Keith, Mario Masellis, Ekaterina Rogaeva, Alexis Brice, Suzanne Lesage, Georgia Xiromerisiou, Andrea Calvo, Antonio Canosa, Adriano Chio, Giancarlo Logroscino, Gabriele Mora, Reijko Krüger, Patrick May, Daniel Alcolea, Jordi Clarimon, Juan Fortea, Isabel Gonzalez-Aramburu, Jon Infante, Carmen Lage, Alberto Lleó, Pau Pastor, Pascual Sanchez-Juan, Francesca Brett, Dag Aarsland, Safa Al-Sarraj, Johannes Attems, Steve Gentleman, John A. Hardy, Angela K. Hodges, Seth Love, Ian G. McKeith, Christopher M. Morris, Huw R. Morris, Laura Palmer, Stuart Pickering-Brown, Mina Ryten, Alan J. Thomas, Claire Troakes, Marilyn S. Albert, Matthew J. Barrett, Thomas G. Beach, Lynn M. Bekris, David A. Bennett, Bradley F. Boeve, Clifton L. Dalgard, Ted M. Dawson, Dennis W. Dickson, Kelley Faber, Tanis Ferman, Luigi Ferrucci, Margaret E. Flanagan, Tatiana M. Foroud, Bernardino Ghetti, J. Raphael Gibbs, Alison Goate, David S. Goldstein, Neill R. Graff-Radford, Horacio Kaufmann, Walter A. Kukull, James B. Leverenz, Qinwen Mao, Eliezer Masliah, Edwin Monuki, Kathy L. Newell, Jose-Alberto Palma, Olga Pletnikova, Alan E. Renton, Susan M. Resnick, Liana S. Rosenthal, Owen A. Ross, Clemens R. Scherzer, Geidy E. Serrano, Vikram G. Shakkottai, Ellen Sidransky, Toshiko Tanaka, Eric Topol, Ali Torkamani, Juan C. Troncoso, Randy Woltjer, Zbigniew K. Wszolek, Sonja W. Scholz.
Contributor Information
Karri Kaivola, Department of Neurology and Translational Immunology Program, University Hospital and Helsinki University, Helsinki, Finland; Neurodegenerative Diseases Research Unit, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892, USA.
Zalak Shah, Neurodegenerative Diseases Research Unit, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892, USA.
Ruth Chia, Laboratory of Neurogenetics, National Institute on Aging, Bethesda, MD 20892, USA.
Sonja W Scholz, Neurodegenerative Diseases Research Unit, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
International LBD Genomics Consortium:
Sandra E Black, Ziv Gan-Or, Julia Keith, Mario Masellis, Ekaterina Rogaeva, Alexis Brice, Suzanne Lesage, Georgia Xiromerisiou, Andrea Calvo, Antonio Canosa, Adriano Chio, Giancarlo Logroscino, Gabriele Mora, Reijko Krüger, Patrick May, Daniel Alcolea, Jordi Clarimon, Juan Fortea, Isabel Gonzalez-Aramburu, Jon Infante, Carmen Lage, Alberto Lleó, Pau Pastor, Pascual Sanchez-Juan, Francesca Brett, Dag Aarsland, Safa Al-Sarraj, Johannes Attems, Steve Gentleman, John A Hardy, Angela K Hodges, Seth Love, Ian G McKeith, Christopher M Morris, Huw R Morris, Laura Palmer, Stuart Pickering-Brown, Mina Ryten, Alan J Thomas, Claire Troakes, Marilyn S Albert, Matthew J Barrett, Thomas G Beach, Lynn M Bekris, David A Bennett, Bradley F Boeve, Clifton L Dalgard, Ted M Dawson, Dennis W Dickson, Kelley Faber, Tanis Ferman, Luigi Ferrucci, Margaret E Flanagan, Tatiana M Foroud, Bernardino Ghetti, J Raphael Gibbs, Alison Goate, David S Goldstein, Neill R Graff-Radford, Horacio Kaufmann, Walter A Kukull, James B Leverenz, Qinwen Mao, Eliezer Masliah, Edwin Monuki, Kathy L Newell, Jose Alberto Palma, Olga Pletnikova, Alan E Renton, Susan M Resnick, Liana S Rosenthal, Owen A Ross, Clemens R Scherzer, Geidy E Serrano, Vikram G Shakkottai, Ellen Sidransky, Toshiko Tanaka, Eric Topol, Ali Torkamani, Juan C Troncoso, Randy Woltjer, Zbigniew K Wszolek, and Sonja W Scholz
References
- 1. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: A systematic review of population and clinical studies. Psychol Med. 2014;44(4):673–683. [DOI] [PubMed] [Google Scholar]
- 3. Chen Y, Wilson L, Kornak J, et al. The costs of dementia subtypes to California Medicare fee-for-service, 2015. Alzheimers Dement. 2019;15(7):899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chia R, Sabir MS, Bandres-Ciga S, et al. ; American Genome Center . Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat Genet. 2021;53(3):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gegg ME, Schapira AHV. The role of glucocerebrosidase in Parkinson disease pathogenesis. FEBS J. 2018;285(19):3591–3603. [DOI] [PubMed] [Google Scholar]
- 7. Jinn S, Drolet RE, Cramer PE, et al. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases alpha-synuclein aggregation. Proc Natl Acad Sci USA. 2017;114(9):2389–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41(12):1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taga M, Petyuk VA, White C, et al. BIN1 protein isoforms are differentially expressed in astrocytes, neurons, and microglia: Neuronal and astrocyte BIN1 are implicated in tau pathology. Mol Neurodegener. 2020;15(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat Rev Neurol. 2019;15(9):501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis AA, Inman CE, Wargel ZM, et al. APOE genotype regulates pathology and disease progression in synucleinopathy. Sci Transl Med. 2020;12(529):eaay3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao N, Attrebi ON, Ren Y, et al. APOE4 exacerbates alpha-synuclein pathology and related toxicity independent of amyloid. Sci Transl Med. 2020;12(529):eaay1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickson DW, Heckman MG, Murray ME, et al. APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology. 2018;91(12):e1182–e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsuang D, Leverenz JB, Lopez OL, et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70(2):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prokopenko I, Miyakawa G, Zheng B, et al. ; BIOS consortium . Alzheimer’s disease pathology explains association between dementia with Lewy bodies and APOE-epsilon4/TOMM40 long poly-T repeat allele variants. Alzheimers Dement (N Y). 2019;5:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schaffert J, LoBue C, White CL3rd, et al. Risk factors for earlier dementia onset in autopsy-confirmed Alzheimer’s disease, mixed Alzheimer’s with Lewy bodies, and pure Lewy body disease. Alzheimers Dement. 2020;16(3):524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raunio A, Kaivola K, Tuimala J, et al. Lewy-related pathology exhibits two anatomically and genetically distinct progression patterns: A population-based study of Finns aged 85. Acta Neuropathol. 2019;138(5):771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- 19. Van der Auwera GA, Carneiro MO, Hartl C, et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43:11.10.11–11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. [DOI] [PubMed] [Google Scholar]
- 21. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. R Core Team . R: A language and environment for statistical computing; 2007. https://www.r-project.org/
- 24. Guerreiro R, Ross OA, Kun-Rodrigues C, et al. Investigating the genetic architecture of dementia with Lewy bodies: A two-stage genome-wide association study. Lancet Neurol. 2018;17(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rongve A, Witoelar A, Ruiz A, et al. GBA and APOE epsilon4 associate with sporadic dementia with Lewy bodies in European genome wide association study. Sci Rep. 2019;9(1):7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cacabelos R, Torrellas C, Fernandez-Novoa L, Lopez-Munoz F. Histamine and immune biomarkers in CNS disorders. Mediators Inflamm. 2016;2016:1924603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benarroch EE, Schmeichel AM, Parisi JE, Low PA. Histaminergic tuberomammillary neuron loss in multiple system atrophy and dementia with Lewy bodies. Mov Disord. 2015;30(8):1133–1139. [DOI] [PubMed] [Google Scholar]
- 28. Minakaki G, Krainc D, Burbulla LF. The convergence of alpha-synuclein, mitochondrial, and lysosomal pathways in vulnerability of midbrain dopaminergic neurons in Parkinson’s disease. Front Cell Dev Biol. 2020;8:580634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hubble JP, Cao T, Hassanein RE, Neuberger JS, Koller WC. Risk factors for Parkinson’s disease. Neurology. 1993;43(9):1693–1697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual-level sequence data are available on dbGaP (accession number: phs001963.v1.p1). The analysis presented here has not been previously published elsewhere.