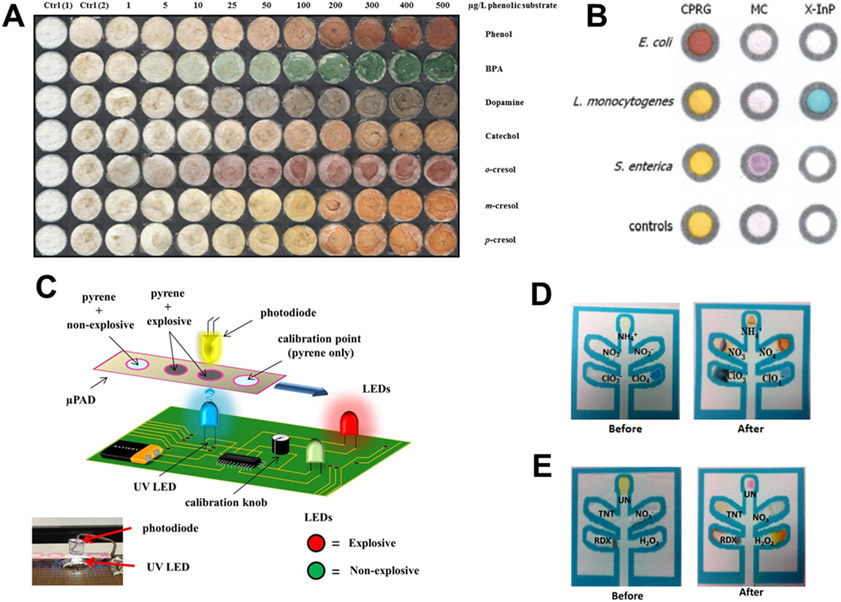
Figure 2:
Examples of μPADs for organic species, bacteria, and explosive compounds. (A) Tyrosinase-based μPAD for detecting phenolic compounds. Reprinted with permission from Ref. 110. Copyright 2012 American Chemical Society. (B) Spot tests for three bacterial strains showing lack of interference. Reprinted with permission from Ref. 141. Copyright 2012 American Chemical Society. (C) Schematic of a handheld fluorescence detector used to measure fluorescence quenching of pyrene by explosive compounds. Reproduced from Ref. 147 with permission from The Royal Society of Chemistry. (D) Colorimetric μPAD for detecting inorganic explosives. Adapted from Ref. 150 with permission from The Royal Society of Chemistry. (E) Colorimetric μPAD for organic and high explosives. Adapted from Ref. 150 with permission from The Royal Society of Chemistry.