Keywords: chrononutrition, intermittent fasting, nutrition, obesity, time-restricted eating
Abstract
Time-restricted eating (TRE) is a dietary intervention that limits food consumption to a specific time window each day. The effect of TRE on body weight and physiological functions has been extensively studied in rodent models, which have shown considerable therapeutic effects of TRE and important interactions among time of eating, circadian biology, and metabolic homeostasis. In contrast, it is difficult to make firm conclusions regarding the effect of TRE in people because of the heterogeneity in results, TRE regimens, and study populations. In this review, we 1) provide a background of the history of meal consumption in people and the normal physiology of eating and fasting; 2) discuss the interaction between circadian molecular metabolism and TRE; 3) integrate the results of preclinical and clinical studies that evaluated the effects of TRE on body weight and physiological functions; 4) summarize other time-related dietary interventions that have been studied in people; and 4) identify current gaps in knowledge and provide a framework for future research directions.
CLINICAL HIGHLIGHTS
The median daily eating window in United States adults is 14 h.
Time-restricted eating (TRE) in people limits daily energy intake to a specific 4- to 12-h period in an effort to optimize cardiometabolic health by leveraging circadian physiology.
In rodents, TRE can prevent and ameliorate the adverse cardiometabolic effects of high-fat and high-sucrose diets.
In people with overweight/obesity, data from randomized controlled trials do not consistently demonstrate clinically important beneficial cardiometabolic effects of TRE (eating window from 4 to 12 h per day).
Further studies are needed to determine whether there are specific features of a TRE regimen (e.g., eating window duration and time of day) that have important cardiometabolic benefits in people.
1. INTRODUCTION
Obesity is associated with a constellation of cardiometabolic abnormalities and diseases, including insulin resistance, β-cell dysfunction, atherogenic dyslipidemia, nonalcoholic fatty liver disease, type 2 diabetes, and cardiovascular disease. The cornerstone of therapy for improving the cardiometabolic complications of obesity is inducing weight loss by consuming fewer calories than expended and mobilizing endogenous adipose tissue stores to meet the body’s energy demands. Many different types of diets that vary in energy content and macronutrient composition have been proposed for treating people with obesity. Meal timing and frequency are two factors that can modulate the relationship between dietary intake, body composition, and metabolic health. In the last 20 years, the potential use of “intermittent fasting” interventions that alter the timing of food consumption to improve metabolic health has become an area of increasing interest and research. Time-restricted eating (TRE), also called time-restricted feeding in animal studies, is a type of intermittent fasting that seeks to leverage circadian physiology to optimize the timing of food intake and improve metabolic health. The purpose of this review is to 1) provide a background of the history of meal timing and frequency in people; 2) review the metabolic responses to eating and fasting; 3) discuss the importance of circadian rhythms in metabolic homeostasis; 4) assess the effects of TRE in rodents and people; and 5) provide suggestions for future research directions.
2. HISTORY OF EATING
2.1. Evolutionary Perspective
Inconsistent food availability had a considerable impact on early hominid and human hunter-gatherer societies. About 2.5 to 1.5 million years ago Homo habilis used primitive stone tools and hunted animals, and ∼500,000 years ago the mastery of fire expanded the dietary repertoire of humans and likely encouraged communal eating (1). The early hunter-gatherers undoubtedly endured periods of fasting and feasting, because food preservation capabilities were limited and access to food depended on opportunistic hunting and fishing and the availability of wild plants (fruits, leaves, and grains), which were the major sources of nutrition during the Paleolithic Era (400,000–40,000 BC) (1–3). Food scarcity was probably a major impetus for the development of the early elements of civilization, including tools, language, and social structures (4, 5), and periods of food scarcity and even famine were general features of hunter-gatherer societies (6, 7).
The introduction of animal domestication and grain farming during the Agricultural Revolution (circa 10,000 BC) decreased the incidence of famine (8) and increased both food availability and the frequency of meal consumption. Ancient Egyptians ate at least three meals daily (1), whereas in ancient Greece people consumed two meals daily (i.e., breakfast and afternoon meal). The afternoon meal was the most important meal of the day and was part of civic life in ancient Greece (1). The Hebrew Bible describes three daily main meals corresponding to a small breakfast or “morning morsel” of bread, a midday meal of bread, dried figs, and wine, and an evening supper that was the largest meal of the day (9). In ancient Rome, breakfast (ientaculum) and lunch (prandium) were informal eating occasions comprised of small amounts of food, whereas the early afternoon coena was the major meal of the day (1). During the Middle Ages in Europe, the number of daily meals gradually increased to three. A breakfast was usually consumed by wealthy people, whereas a meal later in the morning was consumed by the working class. Dinner, the main meal of the day, was consumed during midday, and supper was the last meal of the day and included the consumption of a small quantity of food before twilight (1).
The first Industrial Revolution (1760–1840) was a period when agricultural societies became more industrialized, with migration from farms to cities and major changes in transportation, commerce, manufacturing, food processing, and women’s labor (1). This socioeconomic shift affected the nutritional habits of the population. Breakfast in the morning and lunch in the middle of the working day became established meals, whereas dinner time gradually shifted to much later in the day to accommodate industrial working schedules, long commutes, and increased availability of lighting (1). Work schedules, societal and cultural norms, and availability of highly palatable food continue to shape modern nutritional habits. Data from the National Health and Nutrition Examination Survey (NHANES) indicate that the proportion of Americans eating three meals a day has decreased from 75% to 60% and the proportion consuming >50% of their daily energy intake as snacks has doubled (from 5% to 10%) over the last 40 years (10). Recent food consumption data, collected with a smartphone application, suggest that most (>90%) people consume more than three meals per day and the median time between meals is ∼3 h (11). These results are consistent with NHANES data, which found that American adults have 5.7 eating occasions per day (12). Most people consume food throughout the day; the median daily eating period is ∼14 h, and only 10–15% of adults have a daily eating period of 12 h or less (11, 13). These results underscore a shift in eating behavior in which an increasing proportion of American adults follow an unstructured eating pattern that involves an increase in the number of daily meals and an expansion in the duration of the daily eating period. The timing and frequency of meals also vary among countries. For example, a common eating pattern in Spain is to consume five meals daily, and the main meals of lunch and dinner are consumed ∼2 h later than the corresponding meals in the United Kingdom or the United States (14, 15).
2.2. Religious Fasting
Religion affects food intake during certain times of the year. In Judaism, the Day of Atonement (Yom Kippur) involves a 25-h fast from sunset until nightfall the next day. Christianity has always incorporated fasts, which vary by locality and denomination. Mormons routinely fast for 24 h once every month (16). The 1966 Roman Catholic apostolic constitution Paenitemini established that “fasting” (1 full meal daily without prohibition of snacks) is a form of penitence that is practiced on the holy days of Ash Wednesday and Good Friday (17). Hinduism incorporates ritual fasting that is linked to religious festivals and ranges from abstinence from meat and fish, to liquid dieting, to water-only fasting (18). The practice of fasting in Buddhism is also variable. Monks consume all solid food for the day before noon (19), whereas some lay Buddhists only adhere to this fasting strategy on days of the new or full moon, 6 days per month (20). Occasionally, Buddhist monks engage in prolonged voluntary supervised fasts of 18, 36, or 54 days (20). Fasting is also a common practice of Islam. Some Muslims practice Sunnah fasting, which involves fasting from dawn to dusk on Mondays and Thursdays and holy days (21). Adult Muslims also fast without food or drink from sunrise to sunset during the holy month of Ramadan; the fast duration can vary from 12 to 18 h because the timing of sunrise and sunset varies by season and latitude (22, 23). Traditionally, two meals are consumed during Ramadan: suhoor, which is a small meal eaten before dawn, and iftar, which is a large meal eaten after sunset (24). Some Ramadan fasters will shift their sleep-wake schedule to remain awake into the night, which enables eating more than two meals per night (22, 25). In general, TRE during Ramadan causes weight loss (26–28) followed by weight regain after Ramadan ends (28, 29). Ramadan fasting is also associated with decreased fasting plasma glucose (30–33), inconsistent effects on fasting plasma insulin (29, 34, 35) and plasma lipids (33, 36, 37), and modest decreases in systolic and diastolic blood pressure in normotensive adults (35, 36, 38), without an effect on blood pressure in people with hypertension (31, 39, 40).
3. OVERVIEW OF INTERMITTENT FASTING AND TIME-RESTRICTED EATING
“Intermittent fasting” refers to a group of dietary interventions that limit the timing, rather than the content, of food intake (41). The major types of intermittent fasting regimens that have been studied in people are 1) alternate-day fasting, 2) alternate-day modified fasting, 3) periodic fasting, 4) fasting-mimicking diet (FMD), and 5) time-restricted eating (TABLE 1) (42).
Table 1.
Types of intermittent fasting reported in clinical trials
| Cycle Length | Description | |
|---|---|---|
| Alternate-day fasting | 2 days | Ad libitum intake on day 1, no caloric intake on day 2 |
| Alternate-day modified fasting | 2 days | Ad libitum intake on day 1, ∼25% of daily energy requirement on day 2 |
| Periodic fasting | 1 wk | Ad libitum eating days mixed with calorie-restricted days, e.g., 5:2 with 5 ad libitum eating days and 2 calorie-restricted days/week. Restricted days can be consecutive or nonconsecutive and vary from 0 to 25% of daily energy requirements. |
| Time-restricted eating | 1 day | Caloric intake permitted only within a defined 4- to 12-h window each day. |
Alternate-day fasting, alternate-day modified fasting, and periodic fasting involve restriction of calorie intake (from 0 calories to 25% of daily energy requirements) on “fast” days and ad libitum eating with no restriction of calories on nonfast days (43, 44). Specifically, alternate-day fasting involves consumption of no calories on fast days alternating with unrestricted food intake on nonfast days, alternate-day modified fasting involves consumption of <25% of estimated total energy requirements on fast days alternating with unrestricted food intake on nonfast days, and periodic fasting involves consumption of zero calories to 25% of daily energy requirements on 1 or 2 fast days per week and ad libitum eating on all other days (44). A popular periodic fasting program is the 5:2 diet, which is conducted by fasting (from 0 calories to 25% of daily energy requirements) 2 days per week and eating ad libitum 5 days per week. The 2 fast days can be scheduled on consecutive days (45–48) or nonconsecutive days (49, 50) during the week.
Recently, the effect of treatment with an intermittent low-calorie diet, known as the “fasting-mimicking diet” (FMD), on risk factors for diabetes and cardiovascular disease was reported (51, 52). The FMD is a low-protein (9–10% of total energy), high-fat (44–56% of total energy), low-carbohydrate, calorie-restricted (1,090 kcal on day 1 and 725 kcal on days 2–5) diet that is consumed for 5 consecutive days each month (51, 52). This diet is available commercially; the specific composition of the diet is proprietary.
An important subtype of intermittent fasting that has been studied in both animal models and people is time-restricted eating (TRE). This form of intermittent fasting restricts food intake to a specific duration or window of time each day rather than prescribing fasting days. Moreover, TRE is the only major type of intermittent fasting that does not directly emphasize some form of caloric restriction. Both the duration and the timing of the eating window are important variables that could affect physiological outcomes (53, 54).
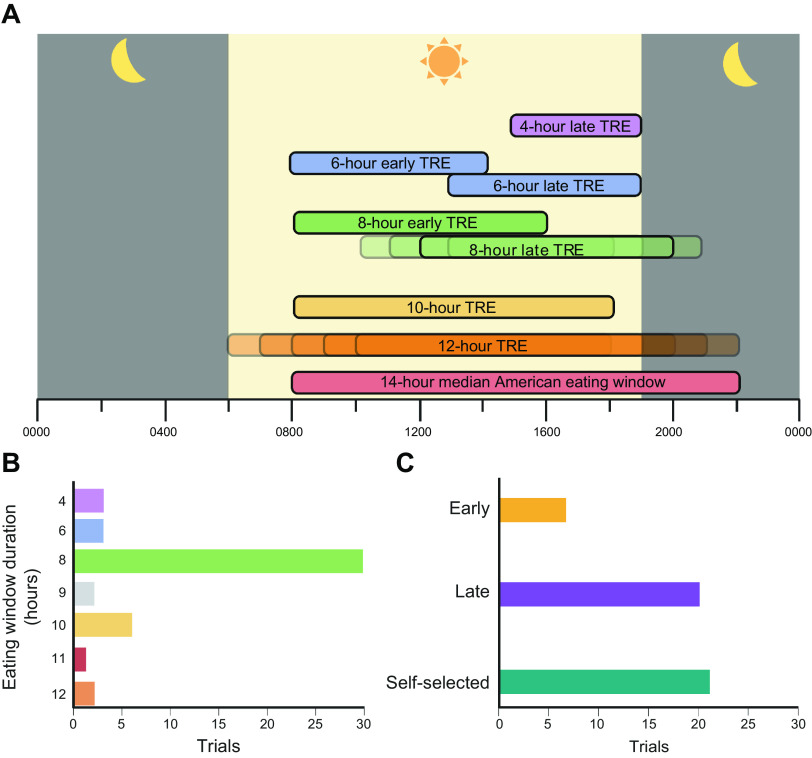
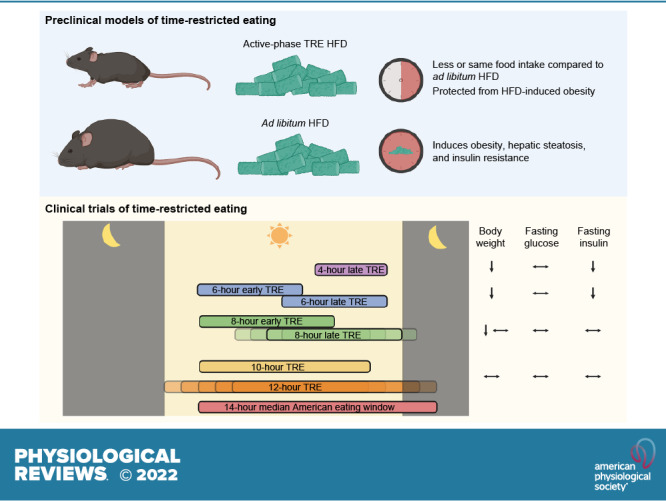
The first study that evaluated the metabolic effects of TRE in people was published in 2015 (11), Since then, many different TRE interventions have been studied (FIGURE 1). The most common eating window duration reported in clinical studies is 8 h (55–84), but the eating window varies considerably among studies and includes durations of 4 h (85, 86), 6 h (85, 87), 9 h (88, 89), 10 h (90–95), and 12 h (11, 96–98). Protocols that initiate the eating window in the early morning and end by 1600 are considered “early” TRE, and those that initiate the eating window later in the morning or in the afternoon and end at 1800 or later are considered “late” TRE. The most common time of day used for the eating window is 1200–2000 (57, 58, 62, 68, 72, 74, 76, 77, 80) but has varied from 0700–1500 (65), to 0800–1600 (59, 66, 83), to 0800–1700 (88), to 0800–1800 (93), to 1000–1800 (56, 73, 80), to 1000–1900 (89), to 1200–2100 (88), to 1300–1900 (85), to 1300–2100 (69, 79), to 1500–1900 (85), to 1930–0330 (70). Some protocols permitted participants to self-select their eating window (60, 61, 63, 64, 67, 71, 75, 78, 81, 90–92, 94–99).
FIGURE 1.
Spectrum of time-restricted eating (TRE) in studies conducted in people. A: the eating window duration in clinical TRE trials ranges from 4 to 12 h, whereas the median American eating window is 14 h. An eating window of 8 h/day from 1200 to 2000 is the most commonly studied TRE protocol. Early TRE windows end at 1600 h or before, whereas late TRE windows end after 1800, permitting an evening meal. B: number of TRE studies conducted in people according to duration of eating window. C: number of TRE studies conducted in people based on early, late, or self-selected eating windows. Figure was created with BioRender.com, with permission.
4. METABOLIC AND ENDOCRINE RESPONSES TO EATING AND FASTING
4.1. Metabolic Response to Eating
There is considerable heterogeneity in the physiological responses to food intake, which are affected by many factors, including insulin sensitivity, intestinal hormones, meal composition, gastric emptying, and time of feeding (100–102). Nonetheless, the overall physiological response to mixed-meal consumption typically involves a switch from fatty acid to glucose oxidation in most tissues, stimulation of hepatic glycogen synthesis and storage, an increase in net skeletal muscle protein synthesis, and an increase in adipose tissue triglyceride storage mediated by both a decrease in adipose tissue lipolytic activity and a concomitant increase in adipose tissue uptake of fatty acids from circulating triglycerides (FIGURE 2) (103, 104).
FIGURE 2.
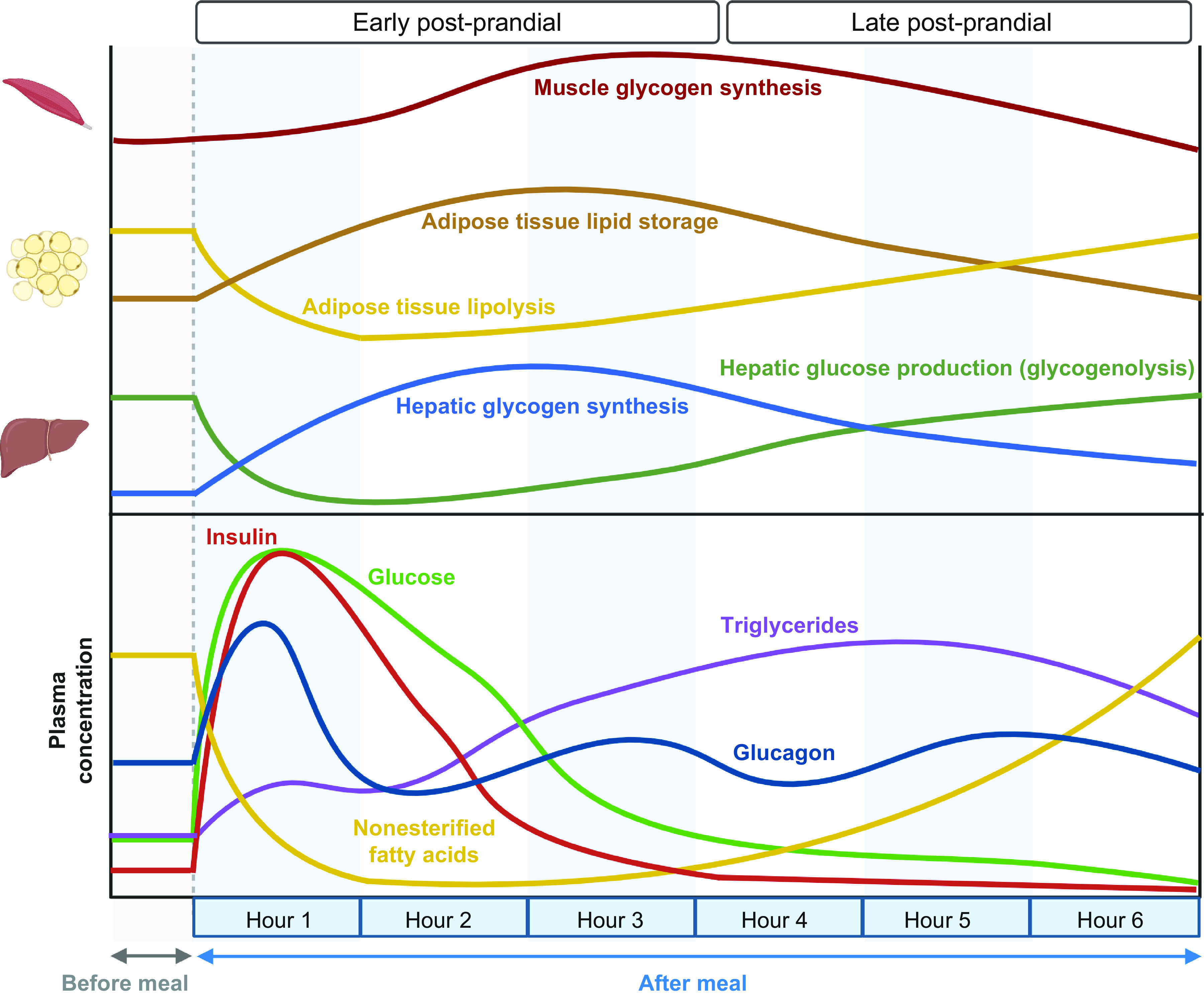
Metabolic response to mixed-meal consumption: effects on organ system physiology and plasma substrate and hormone concentrations. After consumption of a mixed meal, the increase in plasma glucose and incretins stimulates insulin secretion, which in turn suppresses the lipolysis of adipose tissue triglycerides and decreases plasma nonesterified fatty acid concentrations, decreases endogenous glucose production, increases hepatic and muscle glycogen synthesis, and promotes the production of adipocyte triglyceride. The increase in postprandial plasma triglyceride concentration is delayed because of the time needed for the intestine to produce and secrete chylomicrons. Figure was created with BioRender.com, with permission.
Plasma glucose concentration often increases within minutes after meal ingestion, peaks within ∼1 h, and returns to baseline by 3 h postprandially (100, 101, 103, 105). The kinetics of plasma essential amino acid (EAA) concentrations in a mixed meal follow a similar but somewhat delayed pattern, with peak EAA concentrations occurring ∼2 h after meal ingestion and returning to baseline by ∼4 h (103, 106, 107). The postprandial kinetics of dietary triglycerides differ substantially from glucose and amino acids. Ingested triglycerides are hydrolyzed by pancreatic lipases to fatty acids and monoglycerides, which enter the enterocyte as is or as mixed micelles. Short-chain and medium-chain fatty acids and medium-chain triglycerides in the enterocyte are transported into the portal vein. In contrast, long-chain fatty acids are incorporated into chylomicron lipoprotein particles, which are absorbed through the lymphatic system and then delivered into the systemic circulation. Upon meal ingestion, a pool of triglycerides consumed in the previous meal but stored within enterocytes is released into circulation, resulting in a small early rise in plasma triglyceride concentration within 10–30 min (108, 109). For example, this early rise in plasma triglyceride comprises ∼10–15% of dinner triglycerides liberated at the next day’s breakfast (108). Plasma triglyceride concentrations peak ∼3–5 h after meal ingestion and return to baseline ∼8 h later (108, 110, 111).
The consumption of a mixed meal and subsequent intestinal delivery of macronutrients also increases the secretion of incretin hormones [glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)] (112), which regulate the pancreatic secretion of insulin and glucagon (113, 114). Insulin is the primary regulator of the metabolic response to meal ingestion and does so by 1) stimulating glucose disposal, glucose oxidation, and glycogen synthesis in insulin-sensitive tissues (skeletal muscle, adipose tissue, and myocardium) (115, 116); 2) suppressing hepatic glucose production; 3) stimulating de novo lipogenesis in the liver (117–120); 4) promoting muscle protein synthesis (121); 5) suppressing lipolysis and stimulating de novo lipogenesis in adipose tissue (117, 122); and 6) stimulating adipose tissue lipoprotein lipase (LPL) activity, which enhances fatty acid uptake from circulating triglycerides (123). Although both GLP-1 and GIP stimulate insulin secretion, they have opposing effects on the α-cell: GLP-1 inhibits, whereas GIP stimulates, glucagon secretion (124). The typical change in plasma glucagon is much more modest than the change in plasma insulin in response to a mixed-meal concentration (125) and is suppressed after a carbohydrate meal (126). Nonetheless, glucagon is important for maintaining hepatic glucose production and glucose homeostasis after protein ingestion, which stimulates both insulin and glucagon secretion (127, 128).
The metabolic response to meal ingestion is influenced by the time of day at which the meal is consumed and by the previous meal. In healthy people, there is diurnal variation in oral glucose tolerance (129–131); postprandial glycemic excursions after consuming glucose or a mixed meal are greater in the afternoon and evening than in the morning (131–134). This deterioration of glucose tolerance is associated with decreased insulin secretion (133, 134), an increase in plasma nonesterified fatty acid (NEFA) concentrations (129, 135), and a decrease in insulin sensitivity (129–131). The mechanisms responsible for diurnal variation in glucose tolerance are uncertain but could involve the diurnal rhythm of plasma cortisol, because the amplitude of the diurnal variability in plasma cortisol is associated with the magnitude of the diurnal variation in insulin sensitivity (102, 136, 137). In addition, the consumption of a meal can influence postprandial glucose concentrations after the subsequent meal. The term “second-meal phenomenon” describes the observations that 1) the postprandial increase in plasma glucose concentration after a second meal is often less than the postprandial increase in plasma glucose concentration after a similar previous meal and 2) the postprandial increase in plasma glucose concentration is less if a previous meal was eaten than if the first meal was skipped (138–140). After ingestion of glucose or a mixed meal, lipolysis of adipose tissue triglycerides and plasma NEFA concentrations are suppressed, insulin-mediated glucose disposal is increased, and carbohydrate oxidation is increased (140). These factors are believed to “prime” skeletal muscle to increase the uptake of plasma glucose for glycogen synthesis at the next meal (139). The second-meal phenomenon is more pronounced when the intermeal period is short (3–4 h) (139, 141–144) However, there is significant heterogeneity among studies, likely due to competing effects of diurnal variation in glucose tolerance, differences in meal macronutrient composition, sex differences, and differences between people who are normal weight and those with obesity (130, 145–149).
4.2. Metabolic Response to Short-Term Fasting
Fasting triggers a carefully integrated series of metabolic alterations to maintain multiorgan function and enhance survival by 1) decreasing the oxidation of plasma glucose to prevent hypoglycemia, 2) increasing the use of adipose tissue triglycerides as a source of fuel for most tissues, 3) decreasing muscle protein breakdown to conserve lean body mass, and 4) decreasing resting metabolic rate to minimize energy expenditure. The ability of adipose tissue to store and mobilize triglycerides has made it possible for humans to survive periods of food shortage and prolonged fasting. The high energy density and hydrophobic nature of triglyceride make it a fivefold better storage fuel per unit mass than glycogen. Triglycerides are compactly stored as a lipid droplet inside adipocytes and produce ∼9 kcal/g when oxidized, whereas glycogen is stored as a gel, containing ∼2 g of water for every 1 g of glycogen, and produces only ∼4 kcal/g when oxidized. With adequate hydration, the duration of survival during fasting depends on the total amount of adipose tissue. In lean men death occurs after ∼60 days of starvation (150), whereas survival after >1 yr of fasting has been reported in people with severe obesity (151).
In general, there are no adverse consequences of short-term (24–36 h) fasting with adequate hydration, which is proposed in some types of intermittent fasting regimens. During the first 24 h of fasting, there is a decrease in whole body glucose oxidation and an increase in lipolysis of adipose tissue triglycerides and whole body fatty acid oxidation, which occurs primarily between 18 and 24 h (FIGURE 3) (152). Plasma glucose concentration and endogenous glucose production rate remain stable with minimal decline from 12 h to 24 h of fasting, while there is a marked increase in plasma NEFA concentration and lipolytic rate. The rate of hepatic glycogenolysis progressively declines, and the relative contribution of gluconeogenesis (from pyruvate, lactate, alanine, glutamine, and glycerol) to hepatic glucose production increases to help maintain total glucose production rate. At the end of a 24-h fast, most of the 100 g of glucose stored in the liver as glycogen has been mobilized, and only ∼15% of liver glycogen stores remain (153, 154). Whole body glucose oxidation accounts for ∼20% of total energy consumption, whereas whole body fatty acid oxidation accounts for ∼65% of energy consumed during the first 24 h of fasting (152, 155). Moreover, the increase in fatty acid delivery to the liver, in conjunction with an increase in the ratio of plasma glucagon to insulin concentration, increases hepatic ketone body (i.e., β-hydroxybutyrate and acetoacetate) production and begins a shift in brain fuel use from glucose to ketones (102). However, during the first 24 h of a fast, there is only a small increase in plasma ketones to ∼1 mM; a maximal rate of ketogenesis is reached by 3 days of fasting, and plasma ketone body concentration is increased 75-fold by 7 days (156). Approximately 15% of the resting energy requirement is provided by the oxidation of protein; ∼70 g of amino acids is mobilized from protein stores and ∼10 g of nitrogen is excreted in urine during the first 24 h of fasting (157). These early metabolic adaptations to fasting are primarily due to a decrease in carbohydrate, not energy, intake. Providing resting energy requirements during 84 h of fasting by daily infusion of a lipid emulsion does not prevent the normal decrease in plasma glucose and insulin concentrations and increase in plasma fatty acid and ketone body concentrations and lipolytic rate (158).
FIGURE 3.
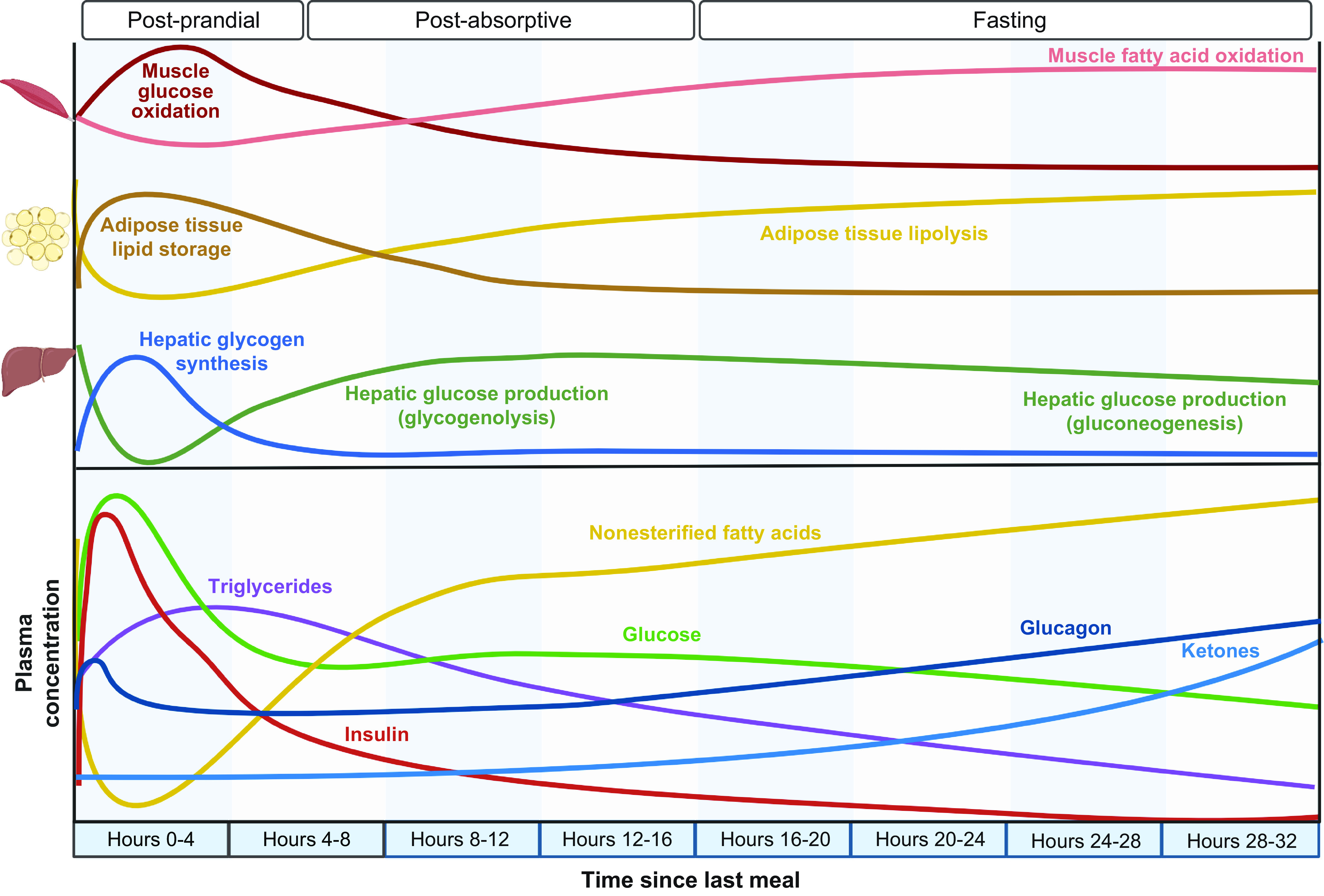
Metabolic response to short-term fasting: effects on organ system physiology and plasma substrate and hormone concentrations. The adaptive response to fasting is triggered by the absence of ingested carbohydrate, which causes a decrease in insulin secretion and an increase in glucagon secretion. These changes in plasma hormones increase lipolysis of adipose tissue triglycerides and cause a shift in whole body fuel consumption from glucose to fatty acids. Plasma glucose concentration is initially primarily maintained by hepatic glycogenolysis followed by hepatic gluconeogenesis. As fasting continues, the increased delivery of fatty acids to the liver in conjunction with alterations in the insulin-to-glucagon ratio stimulates hepatic ketogenesis, which increases plasma ketones (acetoacetate and β-hydroxybutyrate). Figure was created with BioRender.com, with permission.
5. CIRCADIAN RHYTHMS AND METABOLIC HOMEOSTASIS
5.1. Overview of Circadian Biology
Physiological functions and behaviors of mammals, including humans, cycle throughout a 24-h period. These daily rhythms are widespread, from self-evident variations in behaviors such as sleep-wake and fasting-feeding cycles to more subtle and involuntary rhythms such as daily variations in hormones, temperature, blood pressure, muscle tone, and cognitive function. In mammals, these rhythms play an important role in physiology, and their dysfunction contributes to aging and disease (159, 160). The term “circadian” acknowledges the 24-h periodicity of these rhythms and is derived from the Latin circa (about) and diem (day).
Diurnal and circadian rhythms are not the same, even though they are often incorrectly used interchangeably (161). Physiological measures exhibiting a diurnal rhythm have a recurrent period of 24 h under daily cycling conditions and can be generated either endogenously or exogenously (e.g., in response to environmental cues). The term “diurnal” is also used to describe an organism whose activity is highest during daylight. Circadian rhythms, however, must be endogenously generated and satisfy three conditions: 1) continued oscillation when environmental time cues are removed, 2) entrainable by external stimuli, and 3) persist over a range of physiological temperatures (a phenomenon known as temperature compensation) (162).
Circadian rhythms are present in diverse organisms from single-celled cyanobacteria to mammals. The endogenous circadian timekeeping system (CTS) modulates rhythms in physiology and behavior with near 24-h periodicity; light is the main environmental entrainment agent or zeitgeber (“time giver” in German) for the CTS (163). In vertebrates, melanopsin-containing ganglion cells in the retina convey lighting information to the central hypothalamic clock in the suprachiasmatic nucleus (SCN). In turn, the SCN orchestrates circadian rhythms across multiple organ systems, coordinating physiological processes for coherent rhythmic output in physiological and behavioral responses. For example, explants from lung, liver, and other tissues have sustained oscillations in vitro that are temporally aligned with those in SCN explants (164). Because life evolved over billions of years on a revolving planet with ∼24-h periodic variation in light, the circadian clock system presumably evolved to allow organisms to anticipate predictable recurring changes in their environment: to coordinate the timing of core cellular functions with zeitgebers. In this way, biological processes can be activated (e.g., photosynthesis during daylight) or suppressed (e.g., DNA replication during ultraviolet radiation exposure) when most beneficial to the organism (165, 166). In the absence of zeitgebers, cell-autonomous clocks tick according to their endogenous “free running period” or tau (167). The ubiquity of molecular circadian oscillators underscores their significant evolutionary importance to life.
Although nearly all cells possess such circadian clock machinery and the ability to oscillate in a cell-autonomous manner, the process by which the SCN coordinates tissue-specific or “local” peripheral clocks is incompletely understood. Neurohormonal signaling (e.g., through the glucocorticoid receptor) mediates some aspects of central-peripheral clock coordination (168, 169). However, food intake can independently entrain some intestinal and liver clock mechanisms (170–173), suggesting that SCN control of peripheral clocks can be mediated by behavioral patterns (i.e., feeding-fasting cycles) in addition to neurohumoral signaling.
Circadian rhythms can be described by mathematical parameters that define a periodic signal, which facilitates comparisons between groups or interventions (FIGURE 4A). Various tools have been made publicly available to assess whether a biological variable possesses a circadian rhythm (e.g., MetaCycle, JTKCycle, CircWave), and guidelines for the use of these and other tools for analysis of biological rhythms have been published (174, 175). The period of a circadian rhythm is ∼24 h. The amplitude is the difference between peak and trough. A temporal displacement of the signal in response to a stimulus or perturbation is termed a phase shift. Many circadian studies in animals measure activity and rest over the course of multiple days, which can be graphically represented in the form of actograms to allow for easy visualization of the effects of an experimental intervention (FIGURE 4B).
FIGURE 4.
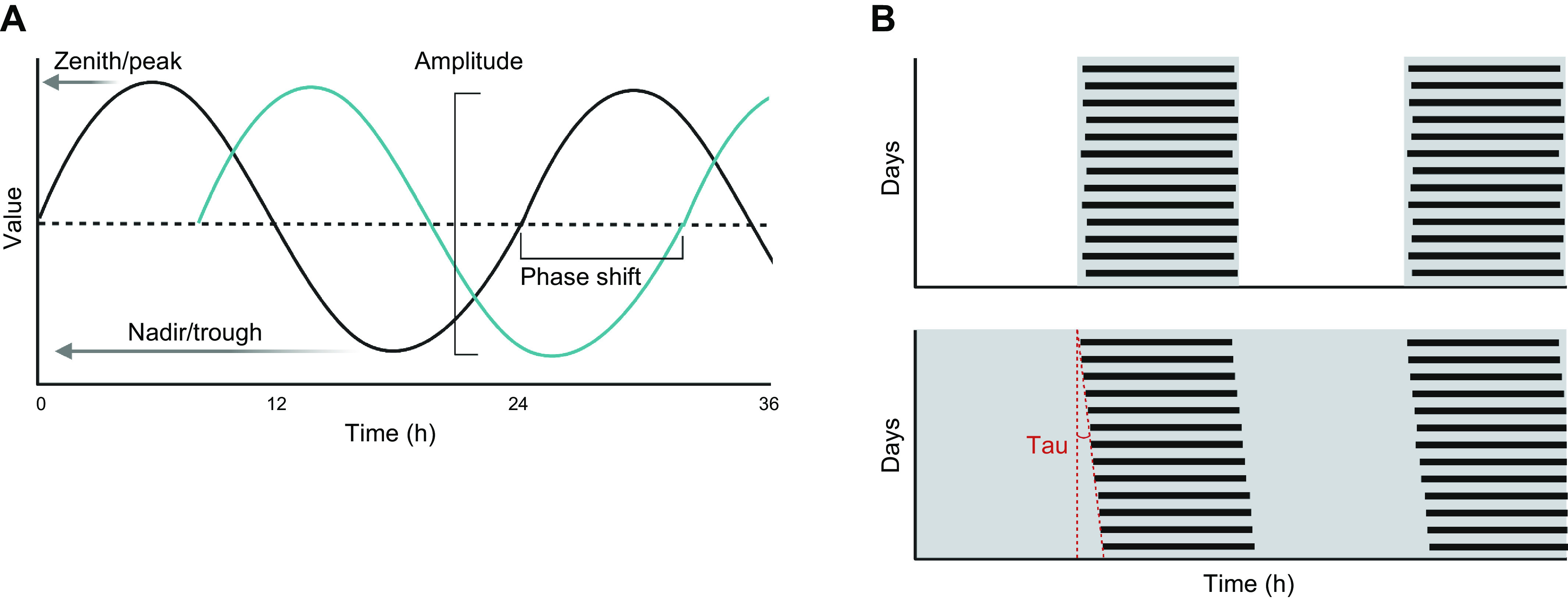
Key variables in circadian biology. A: circadian phase diagram showing key terms related to oscillatory variables, including zenith/peak (the highest value of an oscillating variable), nadir/trough (the lowest value of an oscillating variable), amplitude (the distance from peak to trough value of an oscillating variable), and phase shift (horizontal translation of the circadian oscillation with respect to time). B: example actograms showing an intervention with stable active phase/inactive phase rhythm entrained by a light-dark cycle (top) and progressive shifting (described by the parameter tau) of the active phase/inactive phase with respect to calendar time in a subject with an endogenous circadian period slightly greater than 24 h subjected to a constant routine without light/dark cues (bottom). Figure was created with BioRender.com, with permission.
5.2. Core Circadian Clock Machinery
At the molecular level, the CTS is an autoregulatory transcriptional feedback loop with a period of 24 h that is found in nearly every cell (176, 177). Transcriptional activators of the core clock machinery include the circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like 1 (BMAL1) proteins (161, 178, 179). CLOCK and BMAL1 make up the positive limb of the clock mechanism by forming a heterodimer (CLOCK:BMAL1) that drives rhythmic expression of clock-controlled genes (CCGs) (180). The CLOCK:BMAL1 heterodimer is opposed by the transcriptional repressors period (PER) and cryptochrome (CRY), which comprise the negative limb of this clock mechanism (181).
The downstream targets of CLOCK:BMAL1 include their own repressors PER and CRY, as well as hundreds of other genes (181). Once the positive limb has been activated for a period of time, the PER and CRY proteins accumulate in the cytoplasm to form a complex and migrate to the nucleus to inhibit the CLOCK:BMAL1 heterodimer (181). Posttranslational modification of PERs and CRYs by other regulatory systems [e.g., adenosine monophosphate (AMP)-activated protein kinase (AMPK)] allows fine-tuning of the clock’s speed and oscillatory amplitude (182, 183). In addition to PER and CRY, CLOCK:BMAL1 drives the transcription of the nuclear hormone receptors Rev-Erb and retinoic acid receptor-related orphan receptor (Ror). Together, ROR and REV-ERB orchestrate large-scale cyclical oscillations of hundreds of additional clock-controlled genes, including Bmal1 (184). Accordingly, these autoregulatory feedback loops generate circadian rhythms in much of the transcriptome.
Of the 11,000 ubiquitously expressed genes in primates, nearly all display 24-h rhythmic expression in at least one tissue. Indeed, ∼80% of all protein-coding genes (∼18,000) display circadian expression (185). Furthermore, the majority of genes encoding pharmaceutical drug targets are under circadian control (186). It is therefore not surprising that disruption of circadian rhythms can cause or exacerbate disease.
5.3. Molecular Interactions between the Circadian Clock and Metabolic Regulators
Although metabolic pathways are regulated by the circadian timekeeping system, the clock machinery is itself regulated by metabolic inputs and energy sensors. In particular, many cellular metabolic sensors can posttranslationally modify clock proteins to fine-tune circadian rhythmicity according to the nutritional and energetic status of a cell. This results in a cascade of interactions between metabolic signals [e.g., insulin, IGF-1, amino acids, uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), AMP, nicotinamide adenine dinucleotide (NAD+), and glucagon], metabolic integrators [e.g., AKT, mammalian target of rapamycin (mTOR), O-linked β-N-acetylglucosamine (O-GlcNAc) transferase (OGT), casein kinase 1 (CK1), AMPK, silent information regulator T1 (SIRT1), and cyclic AMP (cAMP) response element-binding protein (CREB)], and clock components (e.g., CLOCK/BMAL1, CRY1/2, PER2) (FIGURE 5). For example, AMPK helps orchestrate the cellular catabolic response to fasting but also modulates the activity of the circadian clock (187). AMPK-mediated phosphorylation during fasting targets CRY for degradation (188), resulting in reduced repression of the CLOCK:BMAL1 complex; this extends the duration of the circadian period. Stabilization of the BMAL1:CLOCK complex through inhibition of ubiquitin-mediated degradation can also be achieved by protein glycosylation by O-linked β-N-acetylglucosamine (O-GlcNAc), an oscillatory intracellular carbohydrate that is sensitive to intracellular glucose levels (189). O-GlcNAcylation also regulates the clock by competing with casein kinase 1 (CK1)-mediated phosphorylation in a serine-rich region of PER, the abundance of which is a key determinant of circadian period and phase (190, 191). O-GlcNAc transferase (OGT), which adds the O-GlcNAc posttranslational modification (PTM) to proteins, is itself regulated by glycogen synthase kinase-3β (GSK3β)-mediated phosphorylation (190, 192). GSK3β plays an important role in regulating circadian rhythmicity (193–198). GSK3β phosphorylates PER2 (194), CRY2 (195), BMAL1 (196), CLOCK (197), and REV-ERBα (198). In general, GSK3β-mediated phosphorylation targets clock proteins to proteasomal degradation. The only exception is REV-ERBα, which is stabilized; this represses Bmal1 and prevents the onset of circadian gene oscillation (198).
FIGURE 5.
Molecular interactions between the clock and metabolic regulators. These physiological relationships can be understood as having 3 main components: metabolic signals, metabolic integrators, and clock integrators. A large number of metabolic signals [e.g., insulin, insulin-like growth factor 1 (IGF-1), amino acids (AA), uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), adenosine monophosphate (AMP), nicotine adenine dinucleotide (NAD+), and glucagon] converge on key metabolic signal integrators [e.g., AKT, mammalian target of rapamycin (mTOR), O-GlcNAc transferase (OGT), AMP-activated protein kinase (AMPK), sirtuin 1 (SIRT1), and CREB (cyclic AMP response element-binding protein (CREB)], which in turn regulate the key components of the circadian timekeeping system [i.e., the circadian locomotor output cycles kaput (CLOCK):brain and muscle ARNT-like 1 (BMAL1) heterodimer and the transcriptional repressors cryptochrome (CRY) and period (PER)]. ATP, adenosine triphosphate; GSK3β, glycogen synthase kinase-3β; mTORC1, mammalian target of rapamycin complex 1; O, O-linked glycosylation; P, phosphorylation, S6K, ribosomal protein S6 kinase. Figure was created with BioRender.com, with permission.
The mammalian target of rapamycin complex 1 (mTORC1) is a master anabolic regulator that controls protein synthesis and inhibits autophagy. There is a bidirectional relationship between mTORC1 and the clock, as the mTORC1 target S6 kinase 1 (S6K1) rhythmically phosphorylates BMAL1 (199), which facilitates BMAL1-mediated control of protein synthesis. On the other hand, the circadian clock itself downregulates mTORC1 activity; Bmal1−/− mice have increased mTORC1 activation (200). mTORC1 also interacts with other circadian proteins. For example, PER2 interacts with regulatory-associated protein of mTOR (RAPTOR) and mTOR to suppress mTORC1 activity (201).
In the fed state, insulin signaling activates AKT/PKB, which in turn regulates diverse anabolic processes including macronutrient metabolism and cell proliferation (202). Activated AKT can phosphorylate cytoplasmic BMAL1 (203) and CLOCK (204), altering the cytoplasmic-nuclear translocation/localization of these proteins and reducing the activity of the circadian clock. Insulin and IGF-1 can also increase PER protein levels via an mTOR-dependent mechanism (172). Finally, AKT phosphorylates and inactivates GSK3β, which as discussed above regulates much of the clock machinery.
Silent information regulator T1 (SIRT1) is another metabolic regulator that interacts with the molecular clock machinery. SIRT1, a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, can upregulate the expression of many genes involved in autophagy and energy metabolism and is a key mediator of the longevity caused by caloric restriction (205). SIRT1 can form a complex with the CLOCK:BMAL1 heterodimer and thus regulate the activity of the circadian clock system (206–208). As part of this complex with CLOCK:BMAL1, SIRT1 can also activate the transcription of the enzyme nicotinamide phosphoribosyltransferase (NAMPT), which leads to its diurnal availability (209, 210). NAMPT is a rate-limiting enzyme in the NAD+ salvage pathway. By causing NAMPT enzyme levels to change rhythmically, SIRT1 induces the circadian oscillation of NAD+. Supplementation with nicotinamide riboside (NR), a precursor of NAD+, increases SIRT1-dependent PER2 deacetylation/destabilization, which in turn strengthens the activity of CLOCK:BMAL1-mediated transcriptional oscillations (211).
The transcription factor cyclic AMP (cAMP) response element-binding protein (CREB) is affected by nutrient availability and has a bidirectional relationship with the clock machinery. In the fed state, CREB activates Per1 transcription (212). This relationship provides a link between G protein-coupled receptors and the regulation of genes in the circadian clock. Although CREB can affect the clock machinery by modulating Per1 expression, CREB can itself be regulated by clock components, as CRY can suppress the activity of CREB (213).
5.4. Genetic Alterations of the Circadian Clock in Rodents
The importance of the CTS in metabolic homeostasis has been shown in mouse models harboring mutations in core circadian clock genes or lacking functional core circadian proteins (TABLE 2). In general, disruption of clock genes in the positive limb of the clock causes metabolic syndrome, obesity, and/or diabetes mellitus. Mice harboring two mutant Clock alleles developed metabolic syndrome, obesity, and defective insulin secretion (214–216). Global Bmal1 deletion results in decreased adipose tissue and skeletal muscle mass and premature aging (227), but tissue-specific Bmal1 deletions have provided insights into tissue-specific clock functions. For example, pancreatic deletion of Bmal1 impairs islet function and causes hyperglycemia (216). Selective deletion of Bmal1 in the brain clock increases food intake during the inactive phase (224), and SCN-specific Bmal1-knockout mice have increased weight gain and fat mass compared to mice with functional Bmal1 (225). However, not all knockouts of Bmal1 have deleterious effects on metabolic health. In fact, deletion of Bmal1 in myeloid cells attenuated the development of atherosclerosis and aortic aneurysm when mice were fed a high-fat diet (HFD) (226). Whole body or SCN-specific knockouts of Rev-erbα/β resulted in increased food intake, body weight, and blood glucose compared to mice with intact Rev-erb signaling (217, 218). Furthermore, whole body knockdown of Rev-erbα/β caused an altered activity pattern with reduced nighttime running, whereas SCN-specific knockdown resulted in increased weight gain, liver triglycerides, fasting blood glucose, and cumulative food intake (218).
Table 2.
Key clock genes associated with metabolic phenotypes in transgenic mice
| Gene | Findings |
|---|---|
| Circadian locomotor output cycles kaput (Clock) | Clock-mutant mice developed obesity, hyperlipidemia, hyperinsulinemia, hyperphagia (214, 215) and hyperglycemia (216). |
| Nuclear receptor subfamily 1 group D member 1/2 (NR1D1/2 Rev-erb α/β) | Whole-body KO mice had increased adiposity and hyperglycemia compared with WT mice (217). SCN-specific KO of Rev-erb α and β results in increased weight gain, higher fasting blood glucose, and increased cumulative food intake under 24-h darkness (218). |
| Period 1/2 (Per1/2) | Per2-mutant (nonfunctional) mice show altered feeding patterns and reduced fasting blood glucose (219) and lack of a food-anticipatory response (220); Per1/2-KO mice show abnormal feeding behavior, consuming 50% of their total intake in the light cycle (221). |
| Cryptochrome 1/2 (Cry 1/2) | Cry1/2-KO mice rapidly gain weight on HFD (222), whereas Cry1-KO mice are protected from weight gain on HFD (223). |
| Brain and muscle ARNT-like 1 (Bmal1) | Brain-clock Bmal1 KO led to an increase in eating during the inactive phase (224); pancreatic Bmal1 KO resulted in impaired insulin secretion and hyperglycemia (216); SCN-specific Bmal1-KO mice gained weight under conditions of constant darkness (225); myeloid-specific Bmal1-KO mice displayed attenuated HFD-induced atherosclerosis (226). |
HFD, high-fat diet; KO, knockout; SCN, suprachiasmatic nucleus; WT, wild type.
Abnormal signaling in the negative limb of the clock also can cause both beneficial and harmful metabolic alterations. For example, mice containing a nonfunctional PER2 protein have reduced fasting blood glucose, which is likely secondary to defects in hepatic glycogen storage (219). Per mutations have also been reported to affect feeding patterns, with mice expressing a mutant Per2 lacking food-anticipatory activity and Per1/2-null mice consuming 50% of their total caloric intake during the light phase rather than the normal 20–30% (220, 221). Studies manipulating Cry1 and Cry2 have also revealed conflicting effects on metabolism; Cry1/2-knockdown mice rapidly gained weight, but Cry1-knockout mice were protected from weight gain upon HFD feeding (222, 223, 228). These studies show that modulation of the circadian clock impacts metabolic homeostasis, though not always in the predicted direction.
5.5. Circadian Biology and Time-Restricted Eating
In rodents, TRE was initially used to study food-anticipatory behavior. Rodents that are fed according to a specific schedule show a bout of activity that precedes the provision of food (229). This food-driven anticipatory behavior generated the hypothesis that food could be used as a zeitgeber. In the early 2000s, several studies showed that food is indeed a zeitgeber that can entrain peripheral expression of clock genes, particularly in the liver (170, 230, 231). In fact, the timing of food intake can cause a complete (i.e., 12 h) phase shift of equal amplitude in the hepatic clock and dampened amplitude in white adipose tissue (173, 232). However, shifting food availability did not shift the phase of clock-gene oscillations in the SCN (170, 230, 231), indicating that the central and peripheral clocks can be desynchronized by providing food only during the inactive phase. In parallel, studies in wild-type (WT) mice revealed that high-fat feeding blunts the normal diurnal oscillations in hepatic Per2, Bmal1, and Rev-erbα expression (233). These results set the stage for investigation of TRE as a preventative and therapeutic intervention for diet-induced obesity in rodents.
Many hepatic transcripts encoding the enzymes of lipid metabolism, glucose metabolism, and cholesterol biosynthesis show circadian regulation (234, 235). Data from knockout mouse models support the importance of the clock in metabolic homeostasis; Clock- or Bmal1-knockout mice have impaired gluconeogenesis after insulin-induced hypoglycemia (236), and Cry1/2−/− mice do not exhibit the normal bimodal rhythm in activity and respiratory exchange ratio (RER) typically observed in WT mice (171). However, when the Cry1/2−/− mice were provided a nocturnal TRE regimen, their RER and food anticipatory activity mirrored that of WT mice (171). Therefore, even in the absence of a functional circadian oscillator, TRE was able to induce these key metabolic rhythms. In addition, interfering with the molecular clock in the liver does not abolish the beneficial effects of TRE; the dysmetabolic phenotypes of liver-specific Bmal1−/− and liver-specific Rev-erbα/β−/− mice were rescued by TRE, and TRE benefits were also observed in Cry1/2−/− mice (228). These results are consistent with the observations that the SCN does not shift in response to inactive-phase TRE (170, 230, 231) and that TRE can still regulate peripheral circadian function in SCN-lesioned mice (225, 231, 237). Together, these observations support the notion that rhythmic feeding/fasting cycles are the major determinant of the phase and rhythmicity of hepatic transcriptional activity, even in the absence of a functional molecular clock (171, 238). It is likely that insulin signaling is involved in regulating these hepatic oscillations because deleting the hepatic insulin receptor prevents the phase shift in hepatic gene expression induced by inactive-phase TRE (232).
5.6. Effect of Time-Restricted Eating on Central Circadian Rhythm in People
Melatonin, measured in saliva, blood, and urine, is a robust marker of central circadian rhythm and circadian phase and period in people when exposure to light is controlled (239–243). Although there are large interindividual differences in the timing and peak of the melatonin rhythm, melatonin levels typically increase ∼2 h before habitual bedtime (244), peak during the night, and return to low levels shortly after habitual waking (245). If exposure to light occurs during the biological night, melatonin levels will be acutely reduced. To accurately assess melatonin levels, samples must be collected every 30–60 min under dim light conditions (e.g., <8 lux in the angle of gaze). Cortisol is another commonly used marker of circadian rhythm in people. During normal sleep/wake and fasting/feeding conditions, cortisol levels peak in the early morning, decrease across the day, remain low near habitual bedtime, and rise after midnight (246, 247). Cortisol release is pulsatile, so accurate assessment of the cortisol rhythm requires fairly frequent sampling (e.g., every 20–30 min).
One study, conducted in young adults with overweight/obesity, found no difference in serial plasma cortisol concentrations obtained for 24 h after 5 days of an 8-h TRE regimen (1000 to 1800) compared with 5 days of a 15-h TRE regimen (0700 to 2200) (65). In contrast, another study that was also conducted in young adults with overweight/obesity found that predinner plasma cortisol concentration was lower after 4 days of a 6-h TRE regimen (0800 to 1400) compared with 4 days of a 12-h TRE regimen (0800 to 2000) (248). Studies that involved longer TRE interventions of >4 wk did not find significant effects of TRE on fasting morning plasma cortisol (63, 67, 87). The effect of meal timing, independently of eating window duration, on central circadian markers has also been studied. One study, conducted in healthy men, involved an intense 13-day inpatient, crossover design, in which factors that could affect circadian rhythms, including sleep time, dim room lighting, and semirecumbent posture, and food intake were kept constant during the 37-h testing period at the end of 6 days of an early (3 meals at 0.5–10.5 h after waking) or a late (3 meals at 5.5–15.5 h after waking) eating schedule (249). This study found no difference in circadian rhythms in either plasma cortisol or melatonin concentrations obtained every hour for 32 h after the early or late eating schedule. Another study that compared the effect of an early eating window (0800 to 1900) with a late eating window (1200 to 2300) for a more prolonged 8-wk period in lean adults found that the diurnal rhythm and circadian parameters (i.e., acrophase and amplitude) for plasma cortisol and melatonin were not different between the early and late eating schedules (250). Overall, the results from these studies indicate that neither TRE nor a shift in the time of the eating window without a change in eating duration affects the central circadian clock in people.
5.7. Effects of Time-Restricted Eating during Circadian Misalignment
Circadian misalignment in people is defined as mistimed behaviors (sleeping, eating/drinking, and physical activity) within the normal 24-h day cycle, for example, sleeping and fasting during the day and waking and eating at night. Circadian misalignment disrupts circadian regulation of the transcriptome and proteome of peripheral blood mononuclear cells (251) in a manner consistent with a disruption of rhythms of proteins known to regulate glucose homeostasis and/or energy metabolism (252). Observational studies of people who work evening, night, or rotating shifts (i.e., “nonstandard” shifts) provide additional insights into the metabolic effects of circadian misalignment. These workers have a higher risk of metabolic (253–256), kidney (257), and cardiovascular (258) disease compared with people who work day shifts. Lifestyle factors such as smoking, decreased physical activity, higher calorie intake, and poor diet quality could be responsible for the increased risk of cardiometabolic disease in people who work nonstandard shifts (259–261). However, several large-scale population studies that attempted to control for lifestyle factors still found an association between shift work and increased disease risk (262). The mechanism for this effect is not clear but is possibly related to eating meals during the biological night, which causes desynchronization of central and peripheral circadian clocks or simply other unrecognized factors inherent to shift work.
Circadian misalignment can be experimentally induced by using a variety of protocols, including the simulated shift work protocol and the forced desynchrony protocol (263). The simulated shift work protocol is designed to mimic patterns found in common night work schedules. The forced desynchrony protocol is conducted under highly controlled conditions with continuous dim light, no external time cues, meals provided at fixed times relative to scheduled wake time, and participants consuming food during both daytime and nighttime hours (239, 240, 264). The results from these protocols consistently show that circadian misalignment causes insulin resistance, decreases oral glucose tolerance (265–267), and can alter the normal circadian rhythmicity of genes involved in DNA repair, increasing the sensitivity to DNA damage (268).
Few studies have evaluated the effect of TRE on metabolic outcomes during circadian misalignment in people, but the available data suggest that TRE attenuates the metabolic dysregulation induced by circadian misalignment. Restricting food intake to the daytime in shift workers attenuated the metabolic abnormalities that occurred when ad libitum eating was allowed overnight (269). In addition, providing meals only during the habitual daytime rather than during the nighttime prevented the adverse effects of experimental simulated night shift work on glucose tolerance and β-cell function (270). Moreover, the amount of food consumed at night during shift work can influence the severity of metabolic dysfunction. In a simulated night shift study, participants who consumed a large snack at midnight had a greater plasma glucose response to breakfast the next day than participants who consumed a small snack at midnight (271). These studies show that TRE during circadian misalignment has beneficial effects on metabolic outcomes.
The attenuation of adverse metabolic effects of circadian misalignment by TRE has also been shown in studies conducted in rodents. In a rat model of shift work, in which exercise was required during the inactive phase, the increase in adiposity and body weight induced by the shift work intervention was attenuated by active-phase TRE (272). Active-phase TRE in mice prevented the glucose intolerance, β-cell dysfunction, and increased adiposity caused by circadian misalignment induced by 8 wk of constant light (273).
6. TIME-RESTRICTED EATING AND ENERGY BALANCE
6.1. Body Weight and Body Composition
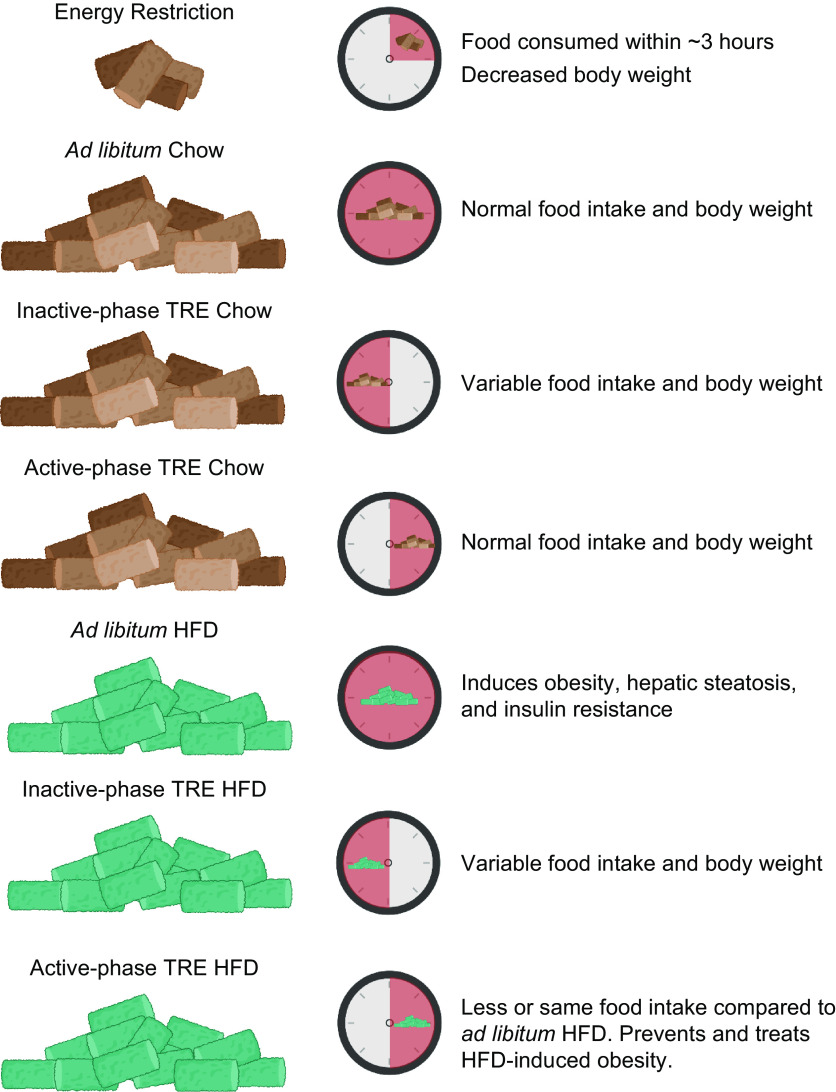
In rodents, the effects of TRE on body weight are influenced by the phase of feeding (light, inactive phase or dark, active phase) and the type of diet provided (FIGURE 6). Restricting mice to HFD feeding in the active phase attenuates or prevents weight gain and adipose tissue accumulation compared to animals with ad libitum access to the same HFD (54, 233, 274–281). Active-phase HFD TRE also causes weight loss in mice previously made obese by HFD feeding (54). The effects of TRE on body weight are less pronounced when animals are fed a normal chow diet or when HFD feeding occurs during the inactive phase. Most studies conducted in chow-fed rodents found no differences in weight gain between ad libitum-fed and active-phase TRE animals (272, 280, 282). However, studies that restricted regular chow intake to the inactive phase show inconsistent effects on body weight, with findings of increased (272, 283), decreased (282), or similar (280) body weight in inactive-phase TRE compared with ad libitum feeding. It is also unclear whether inactive-phase TRE prevents HFD-induced weight gain, because of conflicting results from different studies (274, 276, 280). These studies are confounded by a short feeding period during the inactive phase, which can cause a decrease in total energy intake (282). For example, 4 h of inactive-phase TRE decreased body weight and food intake in both chow-fed and HFD-fed mice compared with ad libitum-fed control mice (276).
FIGURE 6.
Effects of dietary and time-restricted eating (TRE) interventions on cardiometabolic outcomes in mice. Time of feeding is represented by dark pink shading on the clock face. Brown, regular chow. Green, high-fat diet (HFD). Figure was created with BioRender.com, with permission.
In people, 15 randomized controlled clinical trials (RCTs) that were longer than 4 wk in duration evaluated the effect of TRE on body weight in participants who were overweight/obese (TABLE 3). Six of these trials evaluated the effect of TRE on body weight as part of a weight loss program (80, 81, 83, 91, 95, 97). Among these six trials, five prescribed a calorie-deficit diet in both the TRE and control groups (80, 83, 91, 95, 97), whereas one prescribed a calorie-deficit diet in the control group only (81). The duration of the studies ranged from 8 wk to 12 mo, and the length of the eating window in the TRE group ranged from 8 h to 12 h compared with ad libitum consumption of the same dietary prescription in the control group (TABLE 3). A small (1.4–1.7%) but significantly greater weight loss was observed in the TRE group than in the control group in the studies of 8- to 12-wk duration (80, 81, 91). In contrast, none of the three longer duration (9–12 mo) studies observed greater weight loss in the TRE group than in the control group (83, 95, 97).
Table 3.
Randomized controlled trials of TRE with duration ≥4 wk in participants with overweight/obesity
| Study | Participants | Intervention | Duration | Comparator | Weight Change (P value vs. Control) |
Cardiometabolic Outcomes | Adherence |
|---|---|---|---|---|---|---|---|
| Sutton et al. (87) |
N = 8 100% male Age 56 ± 9 BMI 32 ± 4 Prediabetes |
6-h early TRE; 3 meals/day; last meal before 1500 All food provided; weight maintenance attempted |
5 wk | 12-h eating window; 3 meals/day Crossover design |
TRE: −1.4% CON: −1.0% (P > 0.05) |
Increased: plasma TG Decreased: fasting insulin, OGTT mean insulin, total cholesterol, blood pressure No difference: fasting glucose, HOMA-IR, OGTT glucose AUC, HDL-C, LDL-C |
All meals provided and consumption monitored by study staff TRE: 100% of days CON: 99% of days |
| Domaszewski et al. (76) |
N = 45 100% female Age 65 ± 1 BMI 28 ± 1 |
8-h late TRE: ad libitum from 1200 to 2000 | 6 wk | Controls instructed not to change baseline eating habits | TRE: −1.9% CON: +0.8% (P < 0.05) |
Not reported | Participants logged intake Excluded from analysis if adherent <90% of days |
| Peeke et al. (91) |
N = 60 88% female Age 44 ± 1 BMI 39 ± 1 Diabetes excluded |
10-h TRE: commercial weight loss program; given 3 meals, 1 snack to be consumed ad libitum and 1 “fasting snack” to be consumed 2 h before breaking fast daily | 8 wk | 12-h TRE: commercial weight loss program; given 3 meals and 1 snack daily | TRE: −8.5% CON: −7.1% (P < 0.05) |
No difference: fasting glucose | Not reported |
| Kotarsky et al. (58) |
N = 21 86% female Age 44 ± 2 BMI 30 ± 1 Diabetes excluded |
8-h late TRE: ad libitum from 1200 to 2000. Standardized aerobic and resistance training. | 8 wk | Controls instructed not to change baseline eating habits. Standardized aerobic and resistance training. | TRE: −3.7% CON: 0% (P < 0.05) |
No difference: fasting insulin, HbA1c, total cholesterol, HDL-C | Intermittent 3-day dietary records Excluded from analysis if >1 nonadherent day |
| Lin et al. (80) |
N = 63 100% female Age 52 ± 1 BMI 26 ± 1 Diabetes excluded |
8-h TRE: either 1000 to 1800 or 1200 to 2000 1400 kcal guidance |
8 wk | Controls instructed to eat 3 meals daily. 1400 kcal guidance |
TRE: −4.1% CON: −2.4% (P < 0.05) |
Decreased: diastolic blood pressure No difference: fasting glucose, fasting insulin, HOMA-IR, total cholesterol, HDL-C, LDL-C, plasma TG, systolic blood pressure |
Food diaries and/or photography TRE: 84% of days |
| Cienfuegos et al. (85) |
N = 58 90% female Age 47 ± 2 BMI 37 ± 1 Diabetes excluded |
1) 4-h late TRE: ad libitum from 1500 to 1900 2) 6-h late TRE: ad libitum from 1300 to 1900 |
10 wk | Controls instructed not to change baseline eating habits | TRE (4 h): −3.2% TRE (6 h): −3.2% CON: +0.1% (P < 0.05) |
4-h and 6-h TRE: Decreased: fasting insulin, HOMA-IR No difference: fasting glucose, HDL-C, LDL-C, plasma TG |
Food diaries TRE (4 h): 6.2 ± 0.2 days/wk TRE (6 h): 6.2 ± 0.2 days/wk |
| Cai et al. (81) |
N = 271 70% female Age 34 ± 1 BMI 26 ± 1 NAFLD No diabetes medications |
8-h TRE: 1 meal provided, otherwise ad libitum during self-selected eating window | 12 wk | Controls instructed to consume 80% of energy needs | TRE: −4.3% CON: −2.5% (P < 0.05) |
Decreased: plasma TG No difference: fasting glucose, fasting insulin, total cholesterol, HDL-C, LDL-C, blood pressure, liver stiffness |
Unclear Excluded from analysis if nonadherent |
| Lowe et al. (57) |
N = 116 60% male Age 47 ± 1 BMI 33 ± 1 Diabetes excluded |
8-h late TRE: ad libitum from 1200 to 2000 | 12 wk | Controls instructed to eat 3 meals/day at 0600–1000, 1100–1500, and 1700–2200 | TRE: −1.2% CON: −0.8% (P > 0.05) |
No difference: fasting glucose, fasting insulin, HOMA-IR, total cholesterol, HDL-C, LDL-C, plasma TG | Daily self-report adherence surveys TRE: 84% of days CON: 92% of days |
| Chow et al. (60) |
N = 20 85% female Age 46 ± 3 BMI 34 ± 2 >14-h eating window Diabetes excluded |
8-h TRE: ad libitum during a self-selected eating window | 12 wk | Controls instructed not to change baseline eating habits | TRE: −3.8% CON: −1.5% (P < 0.05) |
No difference: fasting glucose, fasting insulin, HOMA-IR, 2-h OGTT glucose, HDL-C, LDL-C, plasma TG | All food photographed in app Mean eating window: TRE: 9.9 h CON: 15.1 h |
| Che et al. (93) |
N = 104 54% male Age 48 ± 1 BMI 26 ± 1 Type 2 diabetes |
10-h TRE: ad libitum from 0800 to 1800 | 12 wk | Controls instructed to maintain baseline diet | TRE: −4.0% CON: −1.1% (P < 0.05) |
Decreased: HbA1c, fasting glucose, fasting insulin, HOMA-IR, plasma TG, total cholesterol, LDL-C No difference: HDL-C |
Food diaries TRE: >6 days/wk |
| Isenmann et al. (77) |
N = 35 50% male Age 28 ± 1 BMI 26 ± 1 |
8-h late TRE: ad libitum from 1200 to 2000 Guidance to eat 45–65% kcal from carbohydrate, 20–35% kcal from fat, 20–35% kcal from protein |
14 wk | Controls instructed to eat diet similar to TRE group | TRE: −4.8% CON: −5.4% (P > 0.05) |
Not reported | Food diaries TRE: 98% of days CON: 89% of days |
| Phillips et al. (98) |
N = 54 Age 43 ± 2 BMI 28 (IQR 25–31) Baseline eating window >14 h, ≥1 metabolic syndrome criterion |
12-h TRE: ad libitum during self-selected eating window | 6 mo | Controls not given specific instructions | TRE: −1.6% CON: –1.1% (P > 0.05) |
No difference: fasting glucose, HbA1c, HDL-C, plasma TG, blood pressure, number of metabolic syndrome criteria met | All food photographed in app Mean eating window: TRE: 12.5 h CON: 14.9 h |
| Thomas et al. (95) |
N = 81 85% female Age 38 ± 1 BMI 34 ± 1 Baseline eating window > 12 h Diabetes excluded |
10-h TRE: ad libitum during self-selected eating window within 3 h of waking, with 35% caloric restriction | 39 wk | Caloric restriction of 35% | TRE: −6.4% (at 12 wk) and −5.1% (at 39 wk) CON: −5.4% (at 12 wk) and −4.6% (at 39 wk) (P > 0.05) |
At 12 wk: No difference: HbA1c, HDL-C, LDL-C, plasma TG, total cholesterol |
Intermittent food photography and questionnaires Mean eating window: TRE: 9.1 h CON: 10.4 h |
| De Oliveira Maranhão Pureza et al. (96, 97) |
N = 58 100% female Age 31 ± 1 BMI 33 ± 1 Diabetes excluded |
12-h TRE, with a 500- to 1,000-kcal deficit prescribed | 12 mo | 500- to 1,000-kcal deficit diet prescribed without time restriction | TRE: −1.7% (at 3 wk) and −0.8% (at 12 mo) CON: −1.1% (at 3 wk) and −0.7% (at 12 mo) (P > 0.05) |
No difference: fasting glucose, fasting insulin (at 3 wk) | Not assessed |
| Liu et al. (83) |
N = 118 51% male Age 32 ± 1 BMI 31 ± 1 Diabetes excluded |
8-h early TRE: ad libitum from 0800 to 1600, with caloric restriction to 1,500–1,800 kcal/day (men) or 1,200–1,500 kcal/day (women) | 12 mo | Caloric restriction to 1,500–1,800 kcal/day (men) or 1,200–1,500 kcal/day (women) | TRE: −9.0% CON: −7.2% (P > 0.05) |
No difference: fasting glucose, HOMA-IR, total cholesterol, HDL-C, LDL-C, plasma TG, blood pressure | All food logged and photographed in app Mean eating window: TRE: 8.1 h CON: 10.9 h |
Age (yr) and body mass index (BMI; kg/m2) are means ± SE for N subjects. Adherence is reported as mean eating window, or by % of adherent days if mean eating window was not described. AUC, area under curve; CON, control; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; OGTT, oral glucose tolerance test; TG, triglyceride; TRE, time-restricted eating.
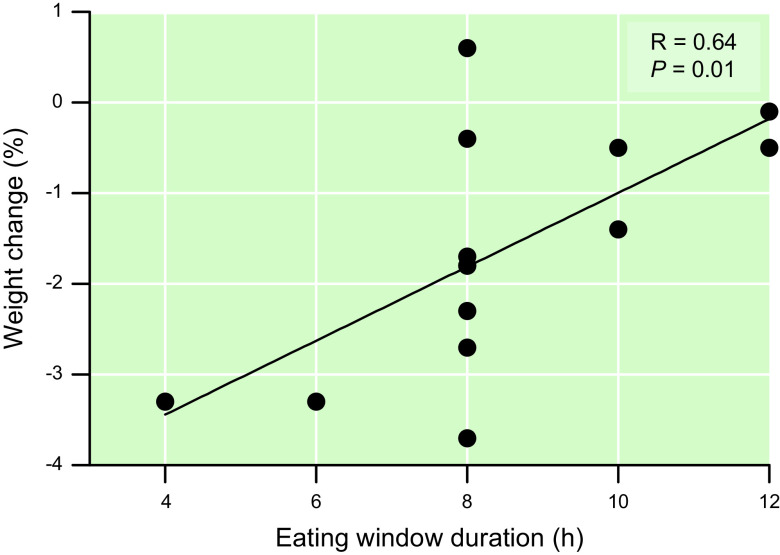
In the nine RCTs that evaluated the effect of TRE on body weight without a concomitant weight loss intervention, TRE caused a small, but not always statistically significant, decrease in body weight compared with the control group (range: 0.6% greater weight to 3.7% lower weight in the TRE group than the control group). The eating window in the TRE groups ranged from 4 h to 12 h, and the duration of the studies ranged from 5 wk to 12 mo. Among all RCTs of TRE of >4-wk duration in people with overweight/obesity and without diabetes, the duration of the eating window correlated with the effect of TRE on percent change in body weight (FIGURE 7). However, there was considerable variability in the change in body weight associated with an 8-h eating window, which was the most common eating window studied.
FIGURE 7.
Relationship between eating window duration and change in body weight in randomized controlled clinical trials of time-restricted eating (TRE) >4 wk in duration that did not attempt weight maintenance and were conducted in participants with overweight/obesity but without diabetes (N = 14 trials). The 4-h and 6-h TRE arms of Ref. 85 are counted separately. Two trials observed a point estimate for weight change of –1.8% with 8-h TRE (81, 83). Weight loss is expressed as the point estimate of mean difference in % weight change between the TRE group and the comparator group.
It is possible that differences among studies in characteristics of the study participants, the intervention prescribed for the active comparator group, variability in the participants’ baseline eating window, adjunctive interventions (e.g., calorie-deficit diets and exercise training), and adherence to the TRE regimen contributed to the heterogeneity in weight loss outcomes. Adherence was assessed in most of the RCTs by intermittent dietary recall, daily food diaries, or logging food intake or meal photographs on a dedicated smartphone application (TABLE 3). Although adherence to the TRE regimen should influence the effect of TRE on body weight, excellent adherence of 80–90% of person-days has been reported both in studies that found greater weight loss in the TRE group compared with the control group and in studies that found no difference in weight change between TRE and control groups (57, 80, 83, 85). Moreover, the measured eating window did not always match the prescribed eating window (60, 90, 92). For example, in one study that observed a 2.3% greater weight loss in the TRE group than the control group, the average eating window, assessed with a smartphone application, was 9.9 h in the group assigned to an 8-h TRE intervention (60). The difference between the duration of the baseline eating window and the prescribed eating window is an additional factor that could influence weight loss outcomes (284), but this hypothesis has not been directly tested.
A series of other studies that evaluated the effect of TRE on body weight in people who were overweight/obese did not include a contemporary control group but assessed the effect of the TRE intervention in comparison to baseline body weight (11, 61, 64, 66, 72, 75, 89, 90, 92, 94, 99), the control group from a previous weight loss study (56), or a nonrandomized control group (74) (TABLE 4). The eating window in the TRE groups ranged from 8 h to 12 h, and the duration of the studies ranged from 4 wk to 16 wk. Weight loss in the TRE group was 0.8–5.5% greater than the change in the comparator values and was significantly different from the comparator in 8 of the 13 studies.
Table 4.
Nonrandomized or uncontrolled trials of TRE with duration ≥4 wk in participants with overweight/obesity
| Study | Participants | Intervention | Duration | Comparator | Weight Change | Cardiometabolic Outcomes | Adherence |
|---|---|---|---|---|---|---|---|
| Anton et al. (61) |
N = 10 60% female Age 77 BMI 34 ± 1 Insulin-dependent diabetes excluded |
8-h TRE: self-selected eating window | 4 wk | Baseline | TRE: −2.2% (P < 0.05) |
No change: fasting glucose | Food diaries TRE: 84% of days |
| Parr et al. (89) |
N = 19 53% female Age 50 ± 2 BMI 34 ± 1 Type 2 diabetes |
9-h TRE: ad libitum from 1000 to 1900 | 4 wk | Baseline | TRE: −0.8% (P > 0.05) |
No change: HbA1c, fasting glucose, fasting insulin, total cholesterol, HDL-C, LDL-C, plasma TG, blood pressure | All food logged and/or photographed in app TRE: 72% of days |
| Kim et al. (72) |
N = 15 60% male Age 37 ± 2 BMI 29 ± 1 Diabetes excluded |
8-h late TRE: 2 meals provided to be eaten between 1200 and 2000. Calories restricted to 1,350 kcal/day | 4 wk | Baseline | TRE (+CR): −4.6% (P > 0.05) |
Decreased: fasting insulin No change: fasting glucose, total cholesterol, LDL-C, plasma TG |
All food provided Daily phone calls to monitor adherence Excluded if nonadherent >2 times in a week |
| Li et al. (66) |
N = 15 100% female Age 18–31 BMI 30 ± 1 Polycystic ovarian syndrome |
8-h early TRE: ad libitum from 0800 to 1600 | 5 wk | Baseline | TRE: −1.7% (P value not reported) |
Decreased: fasting insulin, OGTT insulin AUC, HOMA-IR No change: fasting glucose, OGTT glucose AUC |
Food diaries Adherence not reported |
| Zhao et al. (94) |
N = 15 100% male Age 63 ± 1 BMI 31 ± 1 Diabetes excluded |
10-h TRE: ad libitum during self-selected eating window ending before 1930 | 8 wk | Baseline | TRE: −2.4% (P <0.05) |
Decreased: fasting glucose No change: fasting insulin, blood pressure, total cholesterol, HDL-C, plasma TG |
All food logged and/or photographed in app Mean eating window: 10.6 h |
| Przulj et al. (75) |
N = 50 74% female Age 50 ± 2 BMI 35 ± 1 |
8-h TRE: ad libitum during a self-selected eating window | 12 wk | Baseline | TRE: −3.6% (P < 0.05) |
No change: total cholesterol, LDL-C, HDL-C, plasma TG, blood pressure | Weekly phone calls with self-estimated adherence TRE: 5–6 days/wk |
| Gabel et al. (56) |
N = 23 87% female Age 50 ± 2 BMI 35 ± 1 Diabetes excluded |
8-h TRE: ad libitum from 1000 to 1800 | 12 wk | Historical controls from previous weight loss study | TRE: −2.6% CON: 0.0% (P < 0.05) |
No change: fasting glucose, fasting insulin, HOMA-IR, total cholesterol, HDL-C, LDL-C, plasma TG | Daily logging TRE: 5.6 ± 0.3 days/wk |
| Kesztyüs et al. (64) |
N = 40 78% female Age 49 ± 2 BMI 31 ± 1 Metabolic syndrome |
8- to 9-h TRE: ad libitum, self-selected eating window | 12 wk | Baseline | TRE: −1.9% (P value not reported) |
Decreased: HbA1c No change: total cholesterol, HDL-C, LDL-C, plasma TG |
Food diaries TRE: 86% of days |
| Wilkinson et al. (90) |
N = 19 68% male Age 59 ± 3 BMI 33 ± 1 Metabolic syndrome |
10-h TRE: self-selected eating window | 12 wk | Baseline | TRE: −3% (P < 0.05) |
Decreased: total cholesterol, LDL-C No change: fasting glucose, fasting insulin, HOMA-IR, HDL-C, plasma TG |
All food logged and/or photographed in app Mean eating window: 10.8 h |
| Schroder et al. (74) |
N = 32 100% female Age 38 ± 1 BMI 33 ± 1 |
8-h late TRE: ad libitum from 1200 to 2000 | 3 mo | Controls instructed to continue baseline eating habits | TRE: −4.0% CON: +1.5% (P < 0.05) |
Decreased: systolic blood pressure No change: fasting glucose, fasting insulin, total cholesterol, LDL-C, HDL-C, plasma TG |
Daily text reminders Adherence not assessed |
| Kesztyüs et al. (99) |
N = 63 86% female Age 48 ± 1 BMI 26 ± 1 |
8- to 9-h TRE: ad libitum during a self-selected eating window | 3 mo | Baseline | TRE: −1.8% (P < 0.05) |
Increased: total cholesterol No change: HDL-C, LDL-C, plasma TG |
Food diaries TRE: 72% of days |
| Prasad et al. (92) |
N = 14 75% female Age 51 ± 3 BMI 30 ± 3 Baseline eating window >14 h |
10-h TRE: ad libitum during a self-selected eating window | 3 mo | Baseline | TRE: −1.6% (P < 0.05) |
Decreased: systolic blood pressure No change: diastolic blood pressure |
All food logged and/or photographed in app Mean eating window: 12.0 h |
| Gill and Panda (11) |
N = 8 63% male Age 35 ± 3 BMI 33 ± 2 >14-h eating window No metabolic syndrome or cardiovascular disease |
Self-selected eating window of 10–12 h | 16 wk | Baseline | TRE: −3.4% (P value not reported) |
Not reported | All food logged and/or photographed in app Mean reduction in eating window: 4.5 h |
Age (yr) and body mass index (BMI; kg/m2) are means ± SE (or mean only if SD or SE was not reported) for N subjects. Adherence is reported as mean eating window or by % of adherent days if mean eating window was not described. AUC, area under curve; CON, control; CR, caloric restriction; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; OGTT, oral glucose tolerance test; TG, triglyceride; TRE, time-restricted eating.
The effect of TRE on body weight in participants who were normal weight was evaluated in 13 RCTs (55, 62, 63, 67–69, 71, 73, 78, 79, 84, 86, 250, 285, 286) (TABLE 5). The eating window in the TRE groups ranged from 4 h to 11 h, and the duration of the studies generally ranged from 4 wk to 8 wk, but one study continued for 12 mo (79). One study prescribed a calorie-deficit diet (67), whereas the other studies did not encourage weight loss (55, 62, 63, 68, 69, 73, 78, 79, 84, 86) or even tried to keep body weight constant (71, 250, 285, 286). Seven of the thirteen trials did not detect a significant difference in body weight between the TRE and control group, and three trials found about a 2% significantly greater weight loss in the TRE than the control group (68, 73, 286). Two trials compared early TRE to late TRE and found that participants randomized to early TRE (8 h and 11 h) had minimally greater (∼2%) weight loss than those randomized to late TRE (84, 250). A longer (12 mo) trial that included resistance training in both the 8-h TRE and control groups found no significant differences in weight change between groups at 8 wk but a significant difference in body weight at 12 mo (3.5% weight loss in the TRE group and 3.2% weight gain in the control group) (55, 79). However, only 60% of the participants that were studied at 8 wk completed the 12-mo study, suggesting a potential confounding effect of dropouts on the results.
Table 5.
Randomized controlled trials of TRE with duration ≥4 wk in participants with normal weight
| Study | Participants | Intervention | Duration | Comparator | Weight Change (P value vs. Control) | Cardiometabolic Outcomes |
|---|---|---|---|---|---|---|
| McAllister et al. (63) |
N = 22 100% male Age 22 ± 1 BMI 29 ± 2 |
8-h TRE: ad libitum, self-selected eating window | 4 wk | 8-h TRE: calorie tracking to maintain within 300 kcal/day of baseline | TRE ad libitum: −0.4% (P > 0.05) TRE isocaloric: −0.7% (P > 0.05) |
Increased: HDL-C, adiponectin Decreased: blood pressure No change: fasting glucose, fasting insulin, total cholesterol, LDL-C, plasma TG |
| Stratton et al. (67) |
N = 26 100% male Age 23 ± 1 BMI 26 |
8-h TRE: 25% caloric deficit prescribed | 4 wk | 25% caloric-deficit diet prescribed without time restriction | TRE: −1.5% CON: −1.7% (P > 0.05) |
Not reported |
| Moro et al. (73) |
N = 16 100% male Age 19 ± 1 BMI 22 ± 1 Elite cyclists |
8-h TRE: 3 meals between 1000 and 1800 | 4 wk | 3 meals between 0700 and 2100 | TRE: −1.9% CON: +0.3% (P < 0.05) |
No change: fasting glucose, fasting insulin, total cholesterol, plasma TG |
| Correia et al. (69) |
N = 12 100% male Age 22 ± 1 BMI 24 ± 1 |
8-h late TRE: 2–3 meals from 1300 to 2100 | 4 wk | No meal time restriction Crossover design |
TRE: −0.3% CON: −0.1% (P > 0.05) |
Not reported |
| Tovar et al. (78) |
N = 15 100% male Age 29 ± 1 BMI 23 ± 1 |
8-h TRE: ad libitum during self-selected eating window | 4 wk | 12-h TRE: ad libitum during self-selected eating window Crossover design |
TRE: −1.1% CON: +0.4% (P > 0.05) |
Not reported |
| Xie et al. (84) |
N = 82 78% female Age 31 ± 1 BMI 22 ± 1 |
1) 8-h early TRE: ad libitum between 0600 and 1500 2) 8-h late TRE: ad libitum between 1100 and 2000 |
5 wk | Baseline eating pattern | Early TRE: −2.6% Late TRE: −0.3% CON: +0.5% (P < 0.05 for early TRE vs. CON only) |
Decreased: fasting glucose, HOMA-IR (both early TRE vs. CON only) No change: blood pressure, total cholesterol, LDL-C, HDL-C, plasma TG |
| Martens et al. (71) |
N = 22 55% female Age 67 ± 1 BMI 25 ± 1 |
8-h TRE: self-selected eating window starting between 1000 and 1100, instructed to maintain baseline energy intake | 6 wk | Baseline eating pattern Crossover design |
TRE: −1.1% CON: −1.3% (P > 0.05) |
Increased: total cholesterol, LDL-C Decreased: OGTT glucose AUC No change: fasting glucose, blood pressure, OGTT insulin AUC |
| Allison et al. (250) |
N = 12 58% male Age 26 ± 1 BMI 22 ± 1 |
11-h early TRE: 3 meals and 2 snacks from 0800 to 1900 | 8 wk | 11-h late TRE: 3 meals and 2 snacks from 1200 to 2300 Crossover design |
Early TRE: −1.7% Late TRE: +0.3% (P > 0.05) |
Decreased: HDL-C, HOMA-IR No change: fasting glucose, fasting insulin, total cholesterol, LDL-C, plasma TG |
| Carlson et al. (285) and Stote et al. (286) |
N = 15 67% female Age 45 ± 0.7 BMI 23.4 ± 0.5 Diabetes excluded |
1 meal/day, consumed between 1700 and 2100 Weight maintenance attempted |
8 wk | 3 meals/day Crossover design |
TRE: −0.9% CON: +1.2% (P < 0.05) |
Increased: fasting glucose, OGTT glucose AUC, total cholesterol, HDL-C, LDL-C No change: fasting insulin, HOMA-IR, plasma TG |
| Tinsley et al. (86) |
N = 18 100% male Age 22 ± 1 BMI 25 Resistance-trained men |
4-h TRE: 4 days/wk on nonexercise days; ad libitum intake on exercise days Structured resistance training 3 days/wk |
8 wk | Ad libitum intake; resistance training 3 days/wk. | TRE: −1.1% CON: +3.8% (P > 0.05) |
Not reported |
| Tinsley et al. (62) |
N = 40 100% female Age 22 ± 2 BMI 22 Resistance-trained women |
8-h late TRE: ad libitum from 1200 to 2000 Standardized resistance training |
8 wk | Breakfast at time of waking; ad libitum diet throughout day Standardized resistance training |
TRE: +2.4% CON: +0.0% (P > 0.05) |
No change: fasting glucose, fasting insulin, total cholesterol, HDL-C, LDL-C, plasma TG |
| Brady et al. (68) |
N = 23 100% male Age 38 ± 2 BMI 23 Distance runners |
8-h late TRE: ad libitum from 1200 to 2000 | 8 wk | Controls instructed to maintain eating and exercise habits | TRE: −1% CON: +1% (P < 0.05) |
No change: fasting glucose, insulin, plasma TG |
| Moro et al. (55, 79) |
N = 34 (8 wk) N = 20 (12 mo) 100% male Age 29 ± 4 Resistance-trained men |
8-h late TRE with 3 meals/day at 1300, 1600, and 2000 Standardized resistance training |
Studied at 8 wk and 12 mo | 3 meals/day at 0800, 1300, and 2000 Standardized resistance training |
TRE: −1.2% (at 8 wk); −3.5% (at 12 mo) CON: +0.2% (at 8 wk); +3.2% (at 12 mo) (P < 0.05 at 12 mo) |
Increased: HDL-C, adiponectin Decreased: fasting glucose, fasting insulin, plasma TG, LDL-C, testosterone, IGF-1, leptin No change: total cholesterol |
Age (yr) and body mass index (BMI; kg/m2) are means ± SE (or mean only if SD or SE was not reported) for N subjects. AUC, area under curve; CON, control; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; IGF-1, insulin-like growth factor 1; LDL-C, low-density lipoprotein cholesterol; OGTT, oral glucose tolerance test; TG, triglyceride; TRE, time-restricted eating.
A series of randomized controlled trials evaluated the effect of TRE on body composition, assessed by dual-energy X-ray absorptiometry, in people with overweight or obesity (57, 58, 60, 83, 85, 95, 98). Although these studies differed in the duration and timing of the eating window, duration of the intervention, and whether concomitant exercise training was prescribed, there were no unique effects of weight loss induced by TRE on body fat mass or lean body mass beyond those expected or observed by calorie restriction alone.
The interpretation of the data from studies that evaluated the effect of TRE on body weight in people is complicated because of the short duration of most studies, heterogeneity in the length and time of day of the TRE eating window, differences in compliance with the TRE intervention, confounding effects of differences in body weight and caloric intake targets, and variability in the study population. Nonetheless, the overall data demonstrate that TRE per se causes a small amount of weight loss (1–3%) compared with a control group and is unlikely to provide additional weight loss when combined with a caloric restriction program. Moreover, the amount of weight loss is associated with the duration of the eating window, and 12-h TRE interventions did not produce statistically significant weight loss.
6.2. Energy Intake
In mice, the effect of TRE on energy intake is unclear because some studies have found energy intake to be lower in the TRE group than in the ad libitum-fed control group (275, 276, 280, 287, 288) and others have found energy intake to be unaffected by TRE, despite less weight gain in the TRE group (54, 233, 289). Several studies evaluated the effect of TRE on plasma concentrations of leptin and ghrelin, which are hormones that decrease or stimulate food intake, respectively. Mean daily plasma concentrations of ghrelin were not different in ad libitum-fed animals and mice with feeding restricted to 4 h in the inactive phase (276). However, plasma ghrelin was greater in 8-h or 12-h active-phase TRE HFD-fed mice compared with mice fed with an ad libitum HFD (277). Moreover, active-phase TRE HFD-fed mice, which consume fewer calories than ad libitum HFD-fed mice, showed an anticipatory rise in ghrelin just before dark onset (290). In addition, active-phase TRE prevents HFD-induced hyperleptinemia in most (54, 233, 276, 277) but not all (275) studies. Conversely, inactive-phase TRE caused weight gain and hyperleptinemia compared with active-phase TRE in HFD-fed mice (291).
There is also evidence that energy intake, assessed by food records, and hunger/fullness, assessed by visual analog scales, are affected by TRE in people. Two RCTs, conducted in people with overweight/obesity without concomitant exercise training, found 8 wk of a 4-h, 6-h, or 10-h ad libitum TRE intervention caused about a 500 kcal/day decrease in energy intake compared with about a 100 kcal/day reduction in the control group (85, 93). A smaller decrease in energy intake of ∼200 kcal/day was observed in people with normal weight after 5 wk of an 8-h TRE intervention (84). The effect of TRE on hunger depends on the time of the eating window in relationship to the assessment of hunger. An 8-h late TRE intervention (from 1200 to 2000) resulted in higher early-day hunger but lower late-night (2100 and 2200) hunger compared with a 15-h eating window control intervention (65), and a 6-h early TRE intervention (from 0800 to 1400) resulted in decreased midday hunger and increased fullness at midday (1400) but increased hunger and decreased fullness at late night (2300) compared with a 12-h control intervention (from 0800 to 2000) (292). In contrast, a study that involved an early 6-h TRE intervention (dinner before 3 PM) found no difference in hunger in the morning or evening between the TRE and control groups but an increase in evening fullness in the TRE group (87). The effect of TRE on circulating hormones involved in regulating food intake is inconsistent, and different studies have demonstrated that compared with a control intervention early TRE was associated with decreased fasting morning plasma leptin, ghrelin, and GLP-1 and increased fasting evening peptide tyrosine tyrosine (PYY) concentrations (292), no differences in fasting morning plasma leptin or GLP-1 and no differences in PYY concentrations throughout the day (65), and no differences in fasting morning plasma leptin, GLP-1, and ghrelin concentrations (87).
6.3. Energy Expenditure
The effect of TRE on energy expenditure and locomotor activity in mice is not clear because of conflicting results from different studies finding both increased energy expenditure or locomotor activity (233, 274, 277, 278) and no difference in 24-h energy expenditure (277, 289, 293, 294) or total activity (272, 289, 295, 296) in animals on active-phase TRE compared with ad libitum feeding or inactive-phase TRE. However, there is additional evidence that supports the idea that TRE increases energy expenditure and locomotor activity. Adipose tissue thermogenic capacity (i.e., beiging) manifested by white adipose tissue expression of the thermogenic proteins [peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α) and uncoupling protein-1 (UCP1)] is increased after active-phase TRE (281). In addition, TRE is associated with peak activity levels just before feeding, which remain elevated during the feeding period (233, 291, 297, 298), and mice fed during the inactive-phase increase daytime activity (272, 276, 295, 299).
There is no evidence that TRE affects energy expenditure in people. The results from five studies that evaluated the effect of 6–12 h of TRE found no difference between the TRE and control groups on 1) 24-h energy expenditure, assessed by whole room indirect calorimetry (292); 2) daily total energy expenditure assessed in free-living conditions by the doubly labeled water technique (57); 3) measured physical activity (95); or 4) resting energy expenditure, assessed by indirect calorimetry (59, 79, 95, 96).
7. EFFECT OF TIME-RESTRICTED EATING ON SELECTED PHYSIOLOGICAL SYSTEMS
Most studies evaluating the effect of TRE on physiological systems have been conducted in rodent models that compared time-restricted high-fat feeding with ad libitum high-fat feeding. Therefore, it is often not possible to separate the effects of TRE from the effects of negative energy balance per se on study outcomes. Nonetheless, the results from many studies show that TRE has beneficial effects on multiple organ systems and cardiometabolic variables in rodents (FIGURE 8).
FIGURE 8.
Therapeutic effects of time-restricted eating in rodents made obese by high-fat diet feeding. Figure was created with BioRender.com, with permission.
7.1. Glycemic Control
In mice, TRE can prevent and reverse glucose intolerance and hyperinsulinemia induced by HFD feeding (54, 233, 289). The effects of TRE on plasma glucose and insulin concentrations in mice fed regular chow diets are less pronounced than those in HFD-fed mice (54). In general, the beneficial effects of TRE on glucose metabolism in HFD-fed mice are associated with a decrease in body weight gain. However, TRE protected mice from glucose intolerance induced by high-fructose diet feeding, even though weight gain was similar in mice limited to TRE and mice fed an ad libitum high-fructose diet (54).
Many clinical trials have evaluated the effects of TRE on glycemic control in people, assessed by fasting plasma glucose and insulin concentrations, continuous glucose monitoring (CGM), the homeostasis model assessment of insulin resistance (HOMA-IR), oral glucose tolerance, and hemoglobin A1c (HbA1c) (55, 57, 58, 60, 62–64, 66, 68, 71–73, 75, 79–81, 83–85, 87–89, 91, 93, 95, 96, 98, 99, 250, 285, 300, 301). The eating window in the TRE groups ranged from 4 h to 12 h, and the duration of the studies generally ranged from 4 wk to 12 wk, but one study continued for 6 mo (98) and two for 12 mo (79, 83). Most of these studies were conducted in participants who were overweight/obese and without type 2 diabetes (56–58, 60, 61, 64, 66, 72, 74, 80, 81, 83, 85, 87, 90, 91, 96, 98), two in participants with type 2 diabetes (89, 93), and 11 in participants who were lean (55, 62, 63, 68, 71, 73, 79, 84, 250, 285, 301).
In the controlled studies conducted in people with overweight/obesity without diabetes, no effect of TRE on fasting glucose was observed. Effects of TRE on fasting insulin varied by the duration of the eating window, which ranged from 4 h to 12 h. Three RCTs, which involved an 8-h self-selected eating window (60) or a window from 1200 to 2000 (57, 58), found no difference in fasting plasma insulin concentrations between the TRE and control groups, despite a significantly greater weight loss in the TRE group in two (58, 60) of the trials. In contrast, one RCT of 4-h and 6-h TRE found a significant decrease in fasting plasma insulin concentration and HOMA-IR in the TRE groups compared with the control group, in conjunction with a greater decrease in body weight in the TRE groups (85). Another RCT, in which body weight was not significantly different between treatment groups, found that 6-h early TRE caused a significant decrease in fasting plasma insulin and in mean plasma insulin during an oral glucose tolerance test in the TRE group compared with the control group, without a difference in fasting plasma glucose, HOMA-IR, or plasma glucose during the oral glucose tolerance test between groups (87).
In the studies conducted in people with type 2 diabetes, participants underwent 4 wk of 9-h TRE (89) or 12 wk of 10-h TRE (93). A statistically significant effect of TRE on glycemic control was observed only in the 12-wk trial (93). In that trial, TRE resulted in about a 26 mg/dL decrease in fasting plasma glucose and a 1.5% decrease in hemoglobin A1c, compared with decreases of ∼14 mg/dL and 0.7% in fasting glucose and hemoglobin A1c, respectively, in the control participants who were instructed to maintain their eating habits (93).
Most of the RCTs conducted in people with normal weight found no difference in fasting plasma glucose or insulin concentrations between the TRE group (4–8 wk of an 8-h TRE regimen) and the comparator group (62, 63, 68, 71, 73). However, one study of 8-h late TRE with standardized resistance training in young men found decreases in fasting glucose and fasting insulin at 8 wk and 12 mo of follow-up, in the setting of weight loss (55, 79). Additionally, two studies that compared early TRE to late TRE regimens found that HOMA-IR values decreased after early TRE but not late TRE (84, 250).
Several studies evaluated the effects of short-duration (4–25 days) TRE on 24-h serial blood glucose or interstitial CGM in participants with overweight/obesity (TABLE 6). The results from these studies showed that 1) early 9-h TRE (0800 to 1700) and late 9-h TRE (1200 to 2100) decreased 24-h continuous interstitial glucose area under the curve (AUC) compared with values obtained at baseline (88); 2) 24-h serial plasma glucose and insulin AUC were not different in a 8-h TRE (1000 to 1800) group and a 15-h eating window control group (65); and 3) 24-h continuous interstitial glucose was lower when participants were treated with early 6-h TRE (from 0800 to 1400) compared with when they were assigned to a 12-h (0800 to 2000) control eating window (248, 292).
Table 6.
Short duration (<4 wk) studies that evaluated metabolic effects of time-restricted eating
| Study | Participants | Intervention | Duration | Comparator | Weight Change(P value vs. Control) | Cardiometabolic Outcomes |
|---|---|---|---|---|---|---|
| Jamshed et al. (248) and Ravussin et al. (292) |
N = 11 64% male Age 32 ± 2 BMI 30 ± 1 Diabetes excluded |
6-h early TRE: meals provided at 0800, 1100, and 1400 | 4 days | 12-h eating window, meals provided at 0800, 1400, and 2000 Crossover design |
Not reported | Increased: fasting total cholesterol, HDL-C, LDL-C, and β-hydroxybutyrate Decreased: 24-h interstitial glucose, fasting glucose and insulin No change: 24-h energy expenditure |
| Parr et al. (65) |
N = 11 100% male Age 38 ± 2 BMI 32 ± 1 Diabetes excluded |
8-h TRE: meals provided at 1000, 1300, and 1700 | 5 days | 15-h eating window, meals provided at 0700, 1400, and 2100 Crossover design |
Not reported | Increased: 24-h plasma NEFA Decreased: nocturnal plasma glucose AUC, 24-h plasma C-peptide AUC No change: 24-h AUC for plasma glucose, insulin, TG, GLP-1, leptin, PYY, cortisol |
| Hutchison et al. (88) |
N = 15 100% male Age 55 ± 3 BMI 34 ± 1 Diabetes excluded |
1) 9-h early TRE: ad libitum from 0800 to 1700 2) 9-h late TRE: ad libitum from 1200 to 2100 |
1 wk | Baseline diet Crossover design |
TRE(early): −1.1% TRE(late): −0.8% CON: 0.8% (P > 0.05) |
Decreased: fasting CGM glucose (in early TRE only), mixed-meal tolerance test glucose AUC (in both TRE groups), plasma TG (in both TRE groups) No change: fasting CGM glucose (in late TRE only) |
| Jones et al. (59) |
N = 16 100% male Age 23 ± 1 BMI 24 ± 1 |
8-h early TRE: ad libitum from 0800 to 1600 | 2 wk | Controls prescribed energy restriction to match TRE group; all food provided | TRE: −1.4% CON: −1.6% (P > 0.05) |
Increased: mixed-meal skeletal muscle glucose uptake, Matsuda index of insulin sensitivity No change: 24-h mean CGM glucose, fasting glucose, fasting insulin, plasma TG, plasma NEFA |
| Zeb et al. (70) |
N = 56 100% male BMI 25 ± 1 |
8-h late TRE: ad libitum from 1930 to 0330 | 25 days | Controls instructed to continue regular diet | Not reported | Decreased: total cholesterol, plasma TG No change: LDL-C, HDL-C |
Age (yr) and BMI (kg/m2) are means ± SE for N subjects. AUC, area under curve; CGM, continuous glucose monitor; CON, control; GLP-1, glucagon-like peptide 1; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NEFA, nonesterified fatty acids; PYY, peptide tyrosine tyrosine; TRE, time-restricted eating.
A nonrandomized controlled study evaluated the effects of 2 wk of TRE on insulin sensitivity in normal-weight healthy men (59). In this study, skeletal muscle insulin action was assessed by evaluating forearm glucose uptake and whole body insulin sensitivity was assessed by the Matsuda insulin sensitivity index (302) after participants consumed a liquid mixed meal containing dextrose, casein protein, and cocoa powder. The dextrose and protein contents of each meal were based on the participants’ body weight, so the amounts consumed differed among participants and were slightly less after than before the intervention because both groups lost ∼1 kg of body weight. Both forearm muscle glucose uptake and the Matsuda insulin sensitivity index increased more in the TRE group (both values increased) than in the control group (both values decreased) (59).
In summary, the effect of TRE on glycemic control in people is unclear because of inconsistent results among RCTs, differences in weight loss among studies, and differences in the duration of fasting before assessments of glycemic control were made in the TRE and control groups.
7.2. Hepatic Lipid and Cholesterol Metabolism
Studies conducted in mice show that an 8- to 12-h TRE regimen attenuates or completely prevents the increase in hepatic steatosis induced by ad libitum HFD feeding (233, 289, 303, 304). The therapeutic effect of TRE on intrahepatic triglyceride content was associated with less weight gain, increased hepatic expression of genes involved in fatty acid oxidation, and decreased hepatic expression of genes involved in lipogenesis in the TRE HFD-fed group compared with an ad libitum HFD-fed control group (233, 303, 304). We are not aware of any studies that evaluated the effect of TRE on intrahepatic triglyceride content in people.
Active-phase TRE prevents the marked increase in serum cholesterol concentration that occurs when mice are fed an ad libitum HFD (54, 233, 289). This effect is likely mediated by an increased amplitude and/or shift in the diurnal rhythm in ileal and hepatic expression of genes involved in cholesterol and bile acid biosynthesis with TRE compared with ad libitum HFD feeding (54, 233, 305). In addition, fecal excretion of bile acids is increased in mice treated with an 8-h TRE of a HFD, suggesting that bile acid synthesis from cholesterol is increased, which is supported by decreased sterol and bile acid derivatives in the serum and increased hepatic Cyp7a1 and Cyp7b1 expression (54, 233, 279).
Nearly all of the RCTs conducted in people with overweight/obesity that evaluated the effect of TRE on plasma lipids found that plasma total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides in the TRE group were not different from those in the control group, even when there was a significantly greater decrease in body weight and greater duration of fasting before blood samples were obtained in the TRE group than in the control group (57, 58, 60, 80, 81, 83, 85, 95) (TABLE 3). A study conducted in people with type 2 diabetes found a greater decrease in body weight and a greater decrease in plasma total cholesterol, LDL-cholesterol, and triglyceride in the group randomized to a 10-h TRE than the group randomized to maintain their usual ad libitum diet, without a difference in plasma HDL-cholesterol between groups (93).
7.3. Cardiovascular Function
In healthy people and rodents, blood pressure decreases by 10–20% during the inactive phase (306). This “dip” in blood pressure is often abolished in people with type 2 diabetes and in those with hypertension (307), and loss of the blood pressure dip increases the risk for cardiovascular disease (307, 308). An 8- to 12-h TRE regimen prevented the development of nondipping blood pressure in a db/db mouse model of cardiovascular disease and rapidly restored the inactive-phase dip in ad libitum-fed mice switched to TRE (306). Furthermore, an active-phase TRE intervention decreased systolic blood pressure and mean arterial pressure in HFD-fed rats (309). The data from most (57, 58, 60, 81, 83, 85, 98) but not all (80, 87) RCTs conducted in people with overweight/obesity have shown that TRE does not affect systolic or diastolic blood pressure (TABLE 3).
Few studies have evaluated the effects of TRE on cardiac function. In one study conducted in rats, TRE during the active phase protected against cardiac dysfunction, assessed as global longitudinal cardiac strain and left ventricular desynchrony, induced by hindlimb unloading, a model of microgravity and spaceflight (310). In contrast, TRE in aged mice did not demonstrate important cardiac benefits, assessed by echocardiography (311), suggesting that age influences the effects of TRE on cardiac function. The timing of a TRE intervention can also have electrophysiological effects. In mice, inactive-phase TRE, but not active-phase TRE, increased the length of the R-R and QT intervals (312).
7.4. Sleep
Insufficient sleep causes insulin resistance and other alterations in metabolic function in people (313–315) and is associated with an increased risk for diabetes and obesity comparable to traditional risk factors such as family history of diabetes, obesity, and physical inactivity (253). Many factors, including altered neurohormonal signals, tissue-specific insulin resistance, decreased activity due to fatigue, and increased energy intake have been proposed as mechanisms linking insufficient sleep to metabolic dysfunction (316). Although late-night eating is often associated with insufficient sleep, the results from clinical studies demonstrate that insufficient sleep alone, independent of food intake, is sufficient to impair insulin sensitivity as well as alter hunger and appetite hormones (317–320).
Studies conducted in rodents have demonstrated that TRE can attenuate the negative consequences of sleep disruption. For example, active-phase TRE was sufficient to normalize altered hepatic expression of clock genes associated with sleep disruption (321). In both db/db and C57BL/6 mice, active-phase TRE strengthened, whereas inactive-phase TRE dampened, the diurnal rhythm of sleep (321, 322). Specifically, active-phase TRE increased sleep in the inactive phase and reduced sleep in the active phase without changing the total amount of sleep (322), whereas inactive-phase TRE had the opposite effect: sleep was inappropriately redistributed such that sleep increased during the active phase and decreased during the inactive phase (323). The phenomenon of enhanced diurnal rhythms of sleep has also been observed with active-phase TRE in a mouse model of Huntington disease (324). Similarly, a Drosophila TRE protocol was associated with improved diurnal rhythms of sleep as well as increased total sleep duration compared with ad libitum feeding (325). However, the few studies that evaluated the effect of TRE on sleep in people have not shown any beneficial effects. An 8-wk RCT conducted in people with overweight/obesity found that neither a 4-h nor a 6-h late TRE regimen affected sleep quality, sleep duration, sleep onset latency, or insomnia severity (326). A 5-wk RCT conducted in people with normal weight also found no effects of 8-h early or late TRE on sleep quality (84). Taken together, TRE improves multiple aspects of sleep in preclinical models. Although TRE has not demonstrated beneficial effects on sleep in people, additional studies are needed to fully evaluate this issue.
7.5. Oxidative Stress and Inflammatory Markers
In preclinical studies, TRE decreases 1) skeletal muscle oxidative stress in HFD-fed flies (327), 2) inflammation and oxidative stress in multiple tissues including gut, brain, and liver in mice (328, 329), and 3) plasma concentrations of multiple proinflammatory lipids in HFD-fed mice (54). Few clinical studies have evaluated the effect of TRE on markers of oxidative stress or inflammation. Plasma 8-isoprostane concentration, a marker of oxidative stress, decreased in people with overweight/obesity after treatment with 4-h or 6-h TRE compared with control ad libitum diet (85, 87). An uncontrolled 6-wk study found that a 10-h TRE regimen decreased plasma advanced glycated end products and advanced oxidation protein products (301). In a 1-yr study conducted in healthy men undergoing resistance training, an 8-h TRE intervention decreased serum IL-6, IL-1β, and TNF-α concentrations (79). However, men in this study also lost ∼3.5% body weight, so it is unclear whether reduced inflammation was associated with weight loss or TRE per se. No change in plasma C-reactive protein (CRP) concentration was observed in two studies of 6-h or 8-h TRE (58, 87). Four studies that involved varying lengths and eating durations found that TRE did not affect plasma IL-1β, IL-6, and TNF-α concentrations (70, 85, 87, 301), whereas one study found that 5 wk of an 8-h early TRE regimen decreased plasma IL-8 and TNF-α in normal-weight people (84). The differences in experimental designs and outcomes among studies make it difficult to make any robust conclusions regarding the effect of TRE on markers of inflammation in people.
7.6. Gut Microbiome
Diurnal rhythms in the abundance of specific taxa have been observed in the both the salivary and gut microbiome of animals and people, which can be influenced by host sex, gene expression, and feeding pattern (330–333). Fecal samples collected at different times of the day indicate that the human microbiome is highly dynamic, with cyclical oscillation of many prevalent organisms (334, 335). In addition, bacterially produced metabolites that circulate in the bloodstream, such as secondary bile acids and phenolic compounds, also show diurnal oscillations.
Studies conducted in mice show that disruption of the normal oscillations in the gut microbiome can have adverse effects on intestinal and hepatic circadian rhythms. Germ-free mice have dampened expression of circadian clock genes in the liver (336) and perturbation of the hepatic transcriptome (337). Antibiotic-induced microbiome depletion causes circadian dysregulation of the intestine with reductions in the amplitude of circadian clock genes, as well as associated metabolic disturbances (338, 339). The oscillatory signals from the gut microbiome to enterocytes and hepatocytes include short-chain fatty acids and modified bile acids, which can directly affect the expression of circadian clock genes (330, 331). Changes in dietary intake can also disrupt diurnal rhythms of the gut microbiome. Obesogenic diets such as HFD (279) and milk-fat diet (336) obliterate cyclical oscillations of the fecal, cecal, and ileal microbiome. In a mouse model of colonic polyposis, inactive-phase TRE shifted the colonic clock, caused dysbiosis, and exacerbated alcohol-associated colonic carcinogenesis (340).
In mice, TRE maintains the cycling of the gut microbiome by increasing the number of microbial taxa with diurnal oscillations and the amplitude of their oscillations (279, 336). The specific taxa that are affected by TRE include the Lactobacillus genus and Ruminococcaceae family/Oscillibacter genus (279), which are associated with protection against dysmetabolic conditions (341, 342). Restoration of diurnal patterns of Lactobacillus by TRE could affect bile acid metabolism because these bacteria encode bile salt hydrolase (BSH), an enzyme that deconjugates bile acids (343). Chow-fed mice show oscillations in ileal conjugated and deconjugated bile acid concentrations from the active phase to the inactive phase, which are disrupted by ad libitum HFD but maintained during HFD with active-phase TRE (305). These findings suggest that the timing of food intake regulates ileal BSH activity, providing a potential link between circadian oscillations in the microbiome and host physiology by affecting the interaction between bile acids and the bile acid receptors farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5). In support of this hypothesis, overexpression of BSH in the gut microbiome improved glucose tolerance without affecting body weight in both wild-type and ob/ob mice (344), and studies conducted in people have found that increased BSH in the gut microbiome is associated with the therapeutic response to metformin in type 2 diabetes (345).
Daily oscillations in gut microbial profiles are functionally linked to metabolic health in people. A study that evaluated diurnal oscillations of microbial taxa in several large populations found that there was a loss of diurnal oscillation in the relative abundance of 13 specific taxa in people with type 2 diabetes and that this arrhythmia in the gut microbiome was a significant predictor of the development of type 2 diabetes 5 yr later (346). We are aware of only two clinical trials that examined the effect of TRE on the gut microbiome in people; both found increased microbial richness (α-diversity) in fecal samples obtained from participants after 4–5 wk of an 8-h TRE regimen compared with control subjects instructed to maintain their usual diet (70, 84). Cross-sectional studies in people have found that decreased α-diversity is associated with obesity, insulin resistance, and dyslipidemia (347). However, interventional studies conducted in mouse models have found that marked suppression of α-diversity in antibiotic-induced microbiome-depleted mice (339, 348–350) and germ-free mice (351) improved fasting glucose, glucose tolerance, and insulin sensitivity. Moreover, TRE does not affect the α-diversity of the gut microbiome of HFD-fed mice, despite markedly improving metabolic health (279).
7.7. Nutrient Sensing Pathways
Nutrient and energetic sensing pathways such as the mTORC1 and AMPK signaling pathways oscillate in synchrony with feeding-fasting cycles. mTORC1, which senses amino acids and growth factors (352), is activated by feeding and inactivated by fasting (228). Conversely, AMPK, which senses cellular energetic status, is activated by fasting and inactivated by feeding (188). During TRE in rodents, pronounced oscillations occur in these nutrient sensing pathways, even when key components of the molecular clock are deleted (228). High-amplitude diurnal rhythms in phosphorylated ERK, phosphorylated CREB, and ribosomal protein S6, all downstream readouts of multiple nutrient sensing pathways, are present in livers of HFD-fed mice on TRE but not on ad libitum feeding (228, 233). Similarly, the amplitude of oscillations in hepatic glucokinase and sterol regulatory element-binding protein (SREBP)-1c was enhanced by TRE in HFD-fed mice and was better synchronized to the active phase (228). By contrast, rhythmic expression of the gluconeogenic CREB targets pyruvate carboxylase (Pcx) and glucose-6-phosphatase (G6pc) was blunted by TRE in HFD-fed mice (233), attributed to synergistic effects of oscillations in Cry expression and CREB phosphorylation (171, 213).
In rodents, HFD feeding disrupts or induces phase shifts in hundreds of hepatic transcripts (353) but also induces new oscillations, particularly in peroxisome proliferator-activated receptor-γ (PPARγ) target genes (228, 354). TRE prevents HFD-induced oscillations in hepatic PPARγ expression (54) and thereby reduces expression of multiple downstream lipogenic genes (228). The relative contribution of this mechanism among other mechanisms such as decreased energy intake to the protection from HFD-induced hepatic steatosis observed in TRE mice is uncertain.
In skeletal muscle, TRE also affects metabolic rhythms. Oscillations in maximal mitochondrial respiratory capacity were abolished in rats subjected to inactive-phase TRE compared with active-phase TRE or ad libitum feeding of a regular chow diet (355). This was associated with loss of rhythmic expression of PPARγ coactivator-1α (Ppargc1a), a master regulator of mitochondrial biogenesis (355).
To our knowledge, only one study investigated effects of TRE on nutrient sensing pathways in people. In a 4-day randomized crossover study, afternoon whole blood expression of MTOR and AKT2 and morning expression of SIRT1 were subtly increased by 6-h early TRE compared to a control group with a 12-h eating window (248). The physiological significance of these findings is uncertain, but the results suggest that TRE may increase the oscillatory amplitude of pathways involved in sensing both fasting (e.g., AMPK, SIRT1) and feeding (e.g., mTOR, AKT).
7.8. Autophagy
The dysregulation of autophagy is involved in a variety of diseases and their underlying pathologies. Furthermore, the causal role of autophagy in the metabolic and life span extension benefits of calorie restriction is well established (356). However, the role of autophagy in TRE has not been systematically investigated. In mice undergoing TRE, analysis of the hepatic transcriptome suggests selective activation of autophagy in the liver during the prolonged fasting state, consistent with the diurnal activation of the mTORC1 pathway and the presence of amino acids known to play a role in autophagy signaling (phenylalanine, tryptophan, and leucine) (228). Indeed, findings from work in Drosophila suggest that circadian autophagy might contribute to beneficial effects of TRE on life span in the fly (357). Furthermore, intermittent TRE in Drosophila (20-h fast every other day, from days of life 10 to 40) prolonged life span by 10–20%, which was independent of caloric reduction and insulin signaling, though dependent on a functional circadian clock (357). Autophagy was induced specifically during the active-phase fast, and this time-of-day-specific autophagy was necessary and sufficient to induce increases in life span (357). Time-restricted eating in Drosophila delays the age-related decline in cardiac function (325) and decreases obesity-associated impairments in skeletal muscle structure and function (327), potentially by interacting with the circadian clock, the mitochondrial electron transport chain (ETC), and/or the T-complex protein 1 (TCP-1) ring complex (TRiC)/TCP-1 chaperones (325). An understanding of the effects of TRE on autophagy and translation into experimental systems and eventually people is limited by the lack of autophagy biomarkers. No single assay can yet determine whether autophagy is activated or inactivated, and most methods require an ex vivo tissue sample (358). Forthcoming innovation in methods to measure autophagy, such as autophagic flux in human blood samples (359), is necessary to determine whether the beneficial effects of TRE in people are mediated by autophagy and related systems.
8. EFFECTS OF OTHER TIME-RELATED DIETARY INTERVENTIONS IN PEOPLE
8.1. Skipping Breakfast
The effects of skipping breakfast on body weight, total daily energy intake, and metabolic outcomes have been studied both in single-day laboratory-controlled feeding studies and in longer outpatient interventional studies. In general, short-term laboratory-controlled feeding studies in normal-weight participants have found that skipping breakfast increases energy intake in subsequent meals, but the increase is not enough to fully compensate for the skipped breakfast meal, resulting in decreased total daily energy intake (360–365). The results from a series of studies conducted in healthy normal-weight adults found that skipping breakfast was associated with a 130- to 200-kcal increase in energy intake at lunch but a 200- to 400-kcal decrease in total daily energy intake (361, 362, 364, 365). In children aged 8–10 yr, breakfast skipping did not affect ad libitum energy intake at lunch but resulted in decreased total daily energy intake (363). In a meta-analysis of seven RCTs of breakfast skipping, a statistically significant but clinically modest 0.5-kg decrease in body weight was observed with long-term breakfast skipping interventions (366).
Other studies, limited by small sample sizes and relatively short durations, found mixed and small effects on cardiometabolic variables, including small increases in cholesterol associated with breakfast skipping (367), mild glucose intolerance (368), no changes in plasma glucose, insulin, or triglyceride concentrations (367), and no changes in insulin secretion assessed by measuring urinary C-peptide (368). Furthermore, breakfast skipping was associated with no (369–372) or very small (368) reductions in 24-h energy expenditure, possibly because of the elimination of diet-induced thermogenesis in response to breakfast (373).
In general, studies that compared the effect of an experimental breakfast skipping intervention with a control group show that skipping breakfast induces a small amount of weight loss and has small adverse or no effects on cardiometabolic outcomes (366). These results contradict the data from epidemiological and observational studies that report that breakfast skipping is associated with weight gain (42) and eating breakfast is associated with long-term weight loss maintenance in people with obesity (374).
8.2. Skipping Dinner
We are aware of two studies that evaluated the effect of skipping dinner on metabolic outcomes. The results from these studies are inconsistent, possibly because of differences in study populations. A study conducted in adults with type 2 diabetes found that skipping dinner caused greater weight loss and improvements in fasting plasma glucose, insulin sensitivity, and liver fat content than eating six meals/day (375), whereas a study conducted in healthy normal-weight adults found no changes in plasma glucose or insulin concentrations when dinner was skipped than when three meals were consumed daily (368). There are additional considerations regarding a dinner skipping program that can affect compliance, because of the social and family implications of skipping the traditional group meal of the day and the need to overcome the circadian peak in hunger and appetite that occurs during the biological evening (376).
8.3. Altered Distribution of Energy Intake
Several controlled interventional trials evaluated whether consuming the majority of daily calories in the morning or in the evening has different metabolic effects. The results from most of these trials support the notion that it is better to consume most daily calories in the morning than in the evening (377). In women with overweight/obesity, a low-calorie diet that provided most of the daily calories at breakfast and/or lunch was associated with greater weight loss and decrease in HOMA-IR than the same low-calorie diet that provided most of the daily calories at dinner (378–381). Benefits of consuming a large breakfast have also been observed in people with more profound abnormalities in glucose metabolism and insulin resistance. In adults with type 2 diabetes, providing a large breakfast as part of a low-calorie diet intervention was associated with greater reductions in hemoglobin A1c, blood pressure, and antidiabetic medication use than providing a small breakfast and an isocaloric diet, despite the same decrease in body weight in both treatment groups (382). A study that provided an isocaloric weight-maintenance diet with either a large breakfast or a large dinner to women with polycystic ovarian syndrome found decreases in plasma glucose and insulin during an oral glucose tolerance test after the large-breakfast intervention but not after the large-dinner intervention, without a change in body weight in either group (383). In contrast, a study of men with obesity and insulin resistance who were prescribed a hypocaloric diet with 50% of energy intake consumed at either breakfast or dinner found the decrease in body weight and increase in insulin sensitivity, assessed by the hyperinsulinemic-euglycemic clamp procedure, were not different between the two groups (384).
8.4. One Meal per Day
Consuming only one meal per day is the most restrictive form of TRE. A clinical trial conducted in normal-weight adults randomized participants in a crossover fashion to consuming a single meal under supervision (eaten at one time between 1700 and 2100) or three meals per day for 8 wk, with an 11-wk washout period between the two diet interventions (285, 286). Body weight was measured daily, and energy intake was adjusted as needed to maintain a constant body weight (within 2 kg of baseline) throughout the entire 6-mo study. The one meal/day intervention resulted in increased hunger and desire to eat, decreased fullness, increased fasting plasma glucose and plasma glucose concentrations during an oral glucose tolerance test, and no effect on plasma insulin concentrations. In contrast, a short-term (11 day) randomized crossover trial of one meal/day (eaten between 1700 and 1900) in normal-weight adults found no difference in fasting plasma glucose or insulin concentrations and no difference in the Matsuda index of insulin sensitivity during a mixed-meal tolerance test compared with a three meals/day control intervention, even though the one meal/day intervention resulted in significantly greater weight loss and fat loss (385).
9. CONCLUSIONS AND FUTURE RESEARCH DIRECTIONS
In rodents, active-phase TRE can prevent and treat the adverse cardiometabolic effects of ad libitum HFD feeding, including weight gain and obesity, glucose intolerance, hepatic steatosis, hypercholesterolemia, disruption of the normal oscillations of the gut microbiome, altered sleep rhythms, and ablation of the inactive-phase dip in blood pressure. In contrast, it is difficult to make firm conclusions regarding the effect of TRE in people because of the heterogeneity in results, TRE regimens, and study populations. Nonetheless, the data from RCTs suggest that the beneficial cardiometabolic effects of TRE in people with overweight/obesity are modest (FIGURE 9) and likely influenced by the confounding effects of greater weight loss and a longer duration of fasting before postintervention assessments in the TRE than the comparator groups.
FIGURE 9.
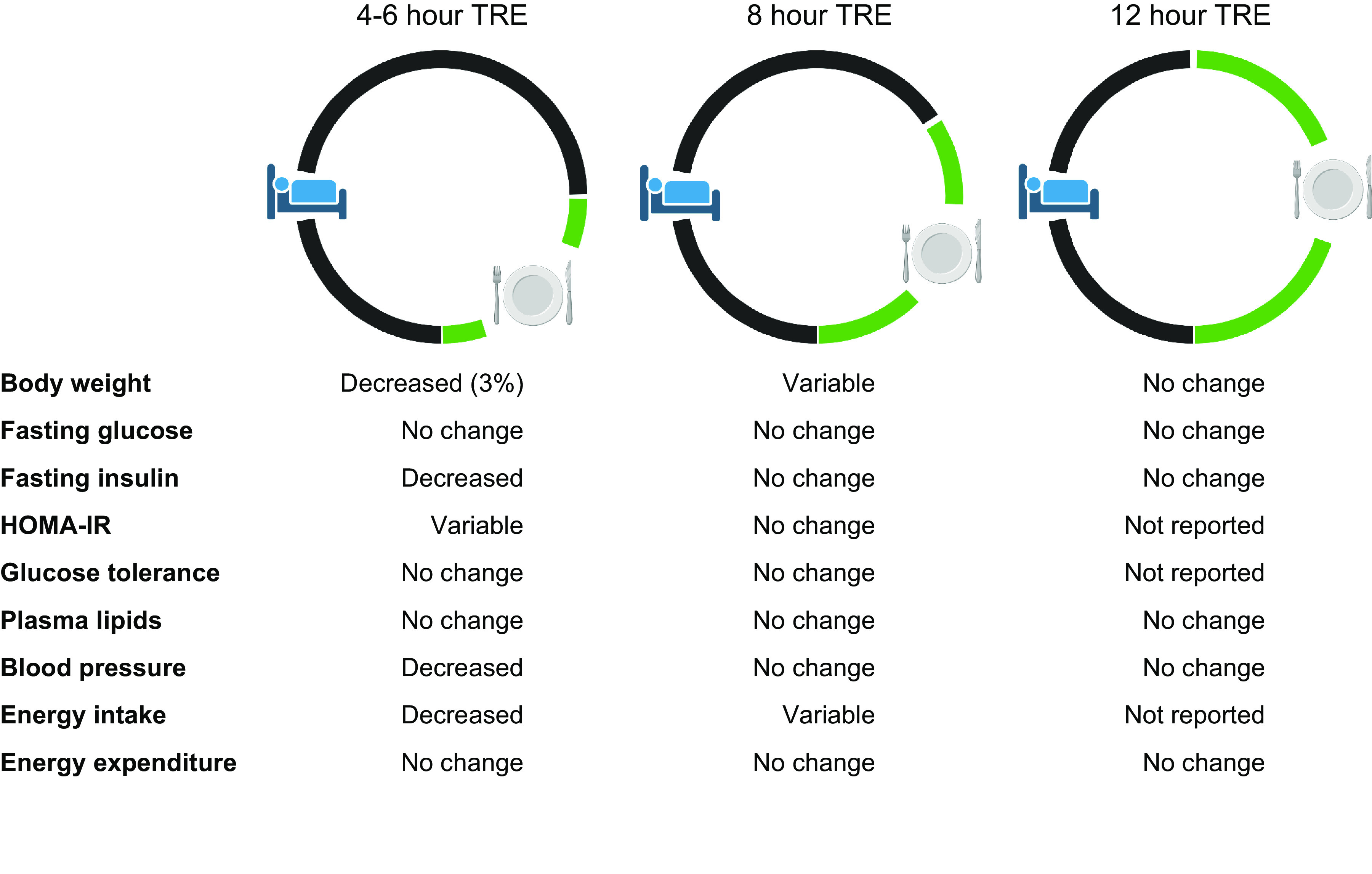
Cardiometabolic effects of different regimens of time-restricted eating (TRE) reported in randomized controlled trials in people with overweight/obesity without diabetes. HOMA-IR, homeostasis model assessment of insulin resistance. Figure was created with BioRender.com, with permission.
The reasons for the discordant cardiometabolic effects of TRE observed in rodents and people are not known but could be related to several factors. First, TRE represents a much greater change in eating behavior in mice than in people. With an ad libitum diet, laboratory mice eat 20–40 times per 24-h day (386), and about one-third of mouse energy intake occurs during the “inactive phase” (386). Second, compliance with the TRE regimen is guaranteed in studies conducted in mice but not in people. Third, the outcome measures (e.g., fasting plasma glucose or insulin) and small sample size in many of the studies conducted in people might not have been sensitive enough to identify small but important metabolic effects. Fourth, most studies conducted in rodents evaluated the effect of TRE within the setting of HFD or high-fat/high-sucrose feeding, whereas the clinical trials of TRE conducted in people did not involve a diet designed to cause weight gain and a deterioration in metabolic function.
The mechanisms responsible for the benefits of TRE in rodents are not completely understood. More research is needed to elucidate the most promising potential mechanisms for the therapeutic effects of TRE, including the importance of 1) oxidative stress, eicosanoid signaling, and inflammatory mediators; 2) modified bile acids and other oscillatory features of the gut microbiome; 3) nutrient sensing pathways, such as AMPK, mTOR, and SIRT1; and 4) autophagy. In addition, the importance of weight loss or prevention of excessive weight gain in inducing the observed benefits of TRE is not clear. Therefore, studies are needed to dissect the influence of differences in body weight on the therapeutic effects of TRE and to better understand the mechanisms responsible for the effect of TRE on food intake. A better understanding of the effect of inactive-phase TRE on metabolic outcomes is also needed, because these studies will help elucidate the metabolic effects of circadian misalignment.
Although a large number of clinical studies have evaluated the cardiometabolic effects of TRE, there are still considerable gaps in our understanding of the potential therapeutic effects of TRE in people that represent important potential directions for future clinical research. Studies are needed to determine whether specific characteristics of a TRE regimen are able to achieve meaningful physiological and clinical beneficial effects. These studies should 1) provide robust measures of cardiometabolic outcomes, such as 24-h blood pressure monitoring, 24-h serial blood sampling to assess important plasma metabolites and hormones, the metabolic response to glucose or mixed-meal ingestion, and rigorous measures of insulin sensitivity; 2) address the potential confounding effects of differences in body weight change and duration of fasting before follow-up testing; 3) determine whether a short (4–6 h) eating window is more therapeutic than a long (8–12 h) eating window; 4) determine whether early (ending before 1600) or late (ending after 1800) TRE has greater cardiometabolic effects; 5) determine whether the metabolic effect of TRE is influenced by dietary macronutrient composition; 6) evaluate the long-term (≥12 mo) effects of TRE; and 7) determine compliance and acceptability of different TRE regimens.
The use of TRE in patients with type 2 diabetes is of concern because it could complicate glycemic management, particularly with short eating windows. Eating windows shorter than 8 h are incompatible with the use of premixed insulin regimens, in which insulin must be taken with food and should be spaced at least 8 h apart to ensure steady provision of basal insulin. Additionally, patients treated with extended-release sulfonylureas or long-acting insulin are at increased risk of hypoglycemia during the fasting period. Therefore, carefully conducted studies with rigorous medical monitoring are needed to evaluate the efficacy and safety of TRE in patients with type 2 diabetes.
The beneficial effects of TRE on specific physiological functions in mice provide a rationale to evaluate these outcomes in people. These include studies to assess the therapeutic effects of TRE on 1) sleep and the adverse effects of experimentally induced sleep disruption and night-shift work; 2) hepatic steatosis and other liver abnormalities in people with nonalcoholic fatty liver disease; and 3) serum cholesterol in people with hypercholesterolemia. In addition, TRE could have synergistic effects with statin therapy. Hepatic hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase expression and cholesterol synthesis have a strong diurnal pattern (387), which is why some statins are most efficacious when taken before sleep. In rodents, HFD feeding alters the diurnal regulation of cholesterol metabolism, which is normalized by TRE with concomitant cholesterol-lowering effects (54), suggesting that TRE combined with statin treatment could be more effective in lowering serum cholesterol concentrations than either treatment alone.
In conclusion, TRE restores circadian oscillations and has potent cardiometabolic benefits in rodents made obese by consuming an ad libitum HFD. However, the effects of TRE in people with overweight/obesity are heterogeneous and modest. The mechanisms responsible for the beneficial effects of TRE in rodents are unclear and require further elucidation. Additional studies are also needed in people to determine the optimal duration and timing of TRE, its effects on metabolic function, and its clinical potential to prevent and treat obesity and cardiometabolic diseases.
GRANTS
We acknowledge support from the Endocrine Fellows Foundation, the Paul and Daisy Soros Foundation, Foundation for Barnes-Jewish Hospital, US Department of Agriculture National Institute of Food and Agriculture (Hatch project no. CA-D-NTR-2618-H), American Heart Association Career Development Awards 852925 and 34110292, VA Merit BLR&D Award I01 BX005707, and National Institutes of Health Grants R01 AG065993, R01 HL148801, R01 EB030134, R01 DK125653, R01 DK115502, P30 DK056341, P30 DK052574, P30 DK020579, P30 DK120515, P30 DK063491, P30 CA014195, P50 AA011999, U01 CA265719, UL1 TR001442, UL1 TR002345, UL1 TR001860, T32 DK110966, and T32 DK007120.
DISCLOSURES
A.Z. is a cofounder and equity-holder in Endure Biotherapeutics. S.K. serves on the Scientific Advisory Board of Altimmune and Merck. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
M.C.P. and S.K. conceived and designed research; M.C.P., M.R.G., S.F.R., A.Z., J.L.B., and A.C. analyzed data; M.C.P., M.R.G., S.F.R., A.Z., J.L.B., M.C., A.C., and S.K. interpreted results of experiments; M.C.P., M.R.G., and A.C. prepared figures; M.C.P., M.R.G., S.F.R., A.Z., J.L.B., M.C., A.C., and S.K. drafted manuscript; M.C.P., M.R.G., S.F.R., A.Z., J.L.B., M.C., A.C., and S.K. edited and revised manuscript; M.C.P., M.R.G., S.F.R., A.Z., J.L.B., M.C., A.C., and S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
Figures were created with BioRender.com.
REFERENCES
- 1. Sonnenfeld A, Flandrin JL, Montanari M. Food: a Culinary History. New York: Columbia University Press, 1999. [Google Scholar]
- 2. Cordain L, Eaton SB, Miller JB, Mann N, Hill K. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr 56, Suppl 1: S42–S52, 2002. doi: 10.1038/sj.ejcn.1601353. [DOI] [PubMed] [Google Scholar]
- 3. Ströhle A, Hahn A, Sebastian A. Estimation of the diet-dependent net acid load in 229 worldwide historically studied hunter-gatherer societies. Am J Clin Nutr 91: 406–412, 2010. doi: 10.3945/ajcn.2009.28637. [DOI] [PubMed] [Google Scholar]
- 4. van Schaik CP, Deaner RO, Merrill MY. The conditions for tool use in primates: implications for the evolution of material culture. J Hum Evol 36: 719–741, 1999. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- 5. Allen JS. “Theory of food” as a neurocognitive adaptation. Am J Hum Biol 24: 123–129, 2012. doi: 10.1002/ajhb.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown PJ, Konner M. An anthropological perspective on obesity. Ann NY Acad Sci 499: 29–46, 1987. doi: 10.1111/j.1749-6632.1987.tb36195.x. [DOI] [PubMed] [Google Scholar]
- 7. Rowland NE. Order and disorder: temporal organization of eating. Behav Brain Res 231: 272–278, 2012. doi: 10.1016/j.bbr.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altmann P, Fu J. Feasting in the Archaeology and Texts of the Bible and the Ancient Near East. Winona Lake, IN: Eisenbrauns, 2014. [Google Scholar]
- 9. King PJ, Stager LE. Life in Biblical Israel. Louisville, KY: Westminster John Knox Press, 2001. [Google Scholar]
- 10. Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet 115: 50–63, 2015. doi: 10.1016/j.jand.2014.06.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 22: 789–798, 2015. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeballos E, Todd JE, Restrepo B. Frequency and Time of Day that Americans Eat: a Comparison of Data from the American Time Use Survey and the National Health and Nutrition Examination Survey. Washington, DC: US Department of Agriculture Economic Research Service, 2019. [Google Scholar]
- 13. Popp CJ, Curran M, Wang C, Prasad M, Fine K, Gee A, Nair N, Perdomo K, Chen S, Hu L, St-Jules DE, Manoogian EN, Panda S, Sevick MA, Laferrère B. Temporal eating patterns and eating windows among adults with overweight or obesity. Nutrients 13: 4485, 2021. doi: 10.3390/nu13124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Southerton D, Diaz-Mendez C, Warde A. Behavioural change and the temporal ordering of eating practices: a UK-Spain comparison. Int J Soc Agric Food 19: 19–36, 2012. [Google Scholar]
- 15. Keller K, Rodríguez López S, Carmenate Moreno MM. Association between meal intake behaviour and abdominal obesity in Spanish adults. Appetite 92: 1–6, 2015. doi: 10.1016/j.appet.2015.04.077. [DOI] [PubMed] [Google Scholar]
- 16. Simmerman SR. The Mormon health traditions: an evolving view of modern medicine. J Relig Health 32: 189–196, 1993. doi: 10.1007/BF00995652. [DOI] [PubMed] [Google Scholar]
- 17. Paul VI. Apostolic Constitution “Paenitemini”. Vatican City: Libreria Editrice Vaticana, 1967. [Google Scholar]
- 18. Knott K. Hinduism: a Very Short Introduction. Oxford: Oxford University Press, 2016. [Google Scholar]
- 19. Keown D. A Dictionary of Buddhism. Oxford: Oxford University Press, 2004. [Google Scholar]
- 20. Gaikwad ST. Apprehending concept, canons and types of fasting in Buddhism. Int J Innov Res Creat Technol 2: 164–168, 2017. [Google Scholar]
- 21. Teng NI, Shahar S, Manaf ZA, Das SK, Taha CS, Ngah WZ. Efficacy of fasting calorie restriction on quality of life among aging men. Physiol Behav 104: 1059–1064, 2011. doi: 10.1016/j.physbeh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 22. BaHammam A, Alrajeh M, Albabtain M, Bahammam S, Sharif M. Circadian pattern of sleep, energy expenditure, and body temperature of young healthy men during the intermittent fasting of Ramadan. Appetite 54: 426–429, 2010. doi: 10.1016/j.appet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 23. Alkandari JR, Maughan RJ, Roky R, Aziz AR, Karli U. The implications of Ramadan fasting for human health and well-being. J Sports Sci 30, Suppl 1: S9–S19, 2012. doi: 10.1080/02640414.2012.698298. [DOI] [PubMed] [Google Scholar]
- 24. Pallayova M, Zaghloul HB, Arora T, Choudhury SM, Omar OM, Chagoury OL, Taheri S. Investigating physiological glucose excursions before, during, and after Ramadan in adults without diabetes mellitus. Physiol Behav 179: 110–115, 2017. doi: 10.1016/j.physbeh.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 25. Hassanein M, Al-Arouj M, Hamdy O, Bebakar WM, Jabbar A, Al-Madani A, Hanif W, Lessan N, Basit A, Tayeb K, Omar M, Abdallah K, Al Twaim A, Buyukbese MA, El-Sayed AA, Ben-Nakhi A; International Diabetes Federation (IDF) and Diabetes and Ramadan (DAR) International Alliance. Diabetes and Ramadan: practical guidelines. Diabetes Res Clin Pract 126: 303–316, 2017. doi: 10.1016/j.diabres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 26. Osman F, Haldar S, Henry CJ. Effects of time-restricted feeding during Ramadan on dietary intake, body composition and metabolic outcomes. Nutrients 12: 2478, 2020. doi: 10.3390/nu12082478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernando HA, Zibellini J, Harris RA, Seimon RV, Sainsbury A. Effect of Ramadan fasting on weight and body composition in healthy non-athlete adults: a systematic review and meta-analysis. Nutrients 11: 478, 2019. doi: 10.3390/nu11020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sadeghirad B, Motaghipisheh S, Kolahdooz F, Zahedi MJ, Haghdoost AA. Islamic fasting and weight loss: a systematic review and meta-analysis. Public Health Nutr 17: 396–406, 2014. doi: 10.1017/S1368980012005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nachvak SM, Pasdar Y, Pirsaheb S, Darbandi M, Niazi P, Mostafai R, Speakman JR. Effects of Ramadan on food intake, glucose homeostasis, lipid profiles and body composition. Eur J Clin Nutr 73: 594–600, 2019. doi: 10.1038/s41430-018-0189-8. [DOI] [PubMed] [Google Scholar]
- 30. Kiyani MM, Memon AR, Amjad MI, Ameer MR, Sadiq M, Mahmood T. Study of human biochemical parameters during and after Ramadan. J Relig Health 56: 55–62, 2017. doi: 10.1007/s10943-015-0084-8. [DOI] [PubMed] [Google Scholar]
- 31. Fakhrzadeh H, Larijani B, Sanjari M, Baradar-Jalili R, Amini MR. Effect of Ramadan fasting on clinical and biochemical parameters in healthy adults. Ann Saudi Med 23: 223–226, 2003. doi: 10.5144/0256-4947.2003.223. [DOI] [PubMed] [Google Scholar]
- 32. Larijani B, Zahedi F, Sanjari M, Amini MR, Jalili RB, Adibi H, Vassigh AR. The effect of Ramadan fasting on fasting serum glucose in healthy adults. Med J Malaysia 58: 678–680, 2003. [PubMed] [Google Scholar]
- 33. Kul S, Savaş E, Öztürk ZA, Karadağ G. Does Ramadan fasting alter body weight and blood lipids and fasting blood glucose in a healthy population? A meta-analysis. J Relig Health 53: 929–942, 2014. doi: 10.1007/s10943-013-9687-0. [DOI] [PubMed] [Google Scholar]
- 34. Gnanou JV, Caszo BA, Khalil KM, Abdullah SL, Knight VF, Bidin MZ. Effects of Ramadan fasting on glucose homeostasis and adiponectin levels in healthy adult males. J Diabetes Metab Disord 14: 55, 2015. doi: 10.1186/s40200-015-0183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shariatpanahi ZV, Shariatpanahi MV, Shahbazi S, Hossaini A, Abadi A. Effect of Ramadan fasting on some indices of insulin resistance and components of the metabolic syndrome in healthy male adults. Br J Nutr 100: 147–151, 2008. doi: 10.1017/S000711450787231X. [DOI] [PubMed] [Google Scholar]
- 36. Jahrami HA, Faris ME, Janahi AI, Janahi MI, Abdelrahim DN, Madkour MI, Sater MS, Hassan AB, Bahammam AS. Does four-week consecutive, dawn-to-sunset intermittent fasting during Ramadan affect cardiometabolic risk factors in healthy adults? A systematic review, meta-analysis, and meta-regression. Nutr Metab Cardiovasc Dis 31: 2273–2301, 2021. doi: 10.1016/j.numecd.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 37. Mirmiran P, Bahadoran Z, Gaeini Z, Moslehi N, Azizi F. Effects of Ramadan intermittent fasting on lipid and lipoprotein parameters: an updated meta-analysis. Nutr Metab Cardiovasc Dis 29: 906–915, 2019. doi: 10.1016/j.numecd.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 38. Ramadan J. Does fasting during Ramadan alter body composition, blood constituents and physical performance? Med Princ Pract 11: 41–46, 2002. doi: 10.1159/000066413. [DOI] [PubMed] [Google Scholar]
- 39. Ural E, Kozdag G, Kilic T, Ural D, Sahin T, Celebi O, Komsuoglu B. The effect of Ramadan fasting on ambulatory blood pressure in hypertensive patients using combination drug therapy. J Hum Hypertens 22: 208–210, 2008. doi: 10.1038/sj.jhh.1002296. [DOI] [PubMed] [Google Scholar]
- 40. Hammoud S, Saad I, Karam R, Abou Jaoude F, van den Bemt BJ, Kurdi M. Impact of Ramadan intermittent fasting on the heart rate variability and cardiovascular parameters of patients with controlled hypertension. J Nutr Metab 2021: 6610455, 2021. doi: 10.1155/2021/6610455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381: 2541–2551, 2019. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 42. St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K; American Heart Association Obesity Committee of the Council on Lifestyle, Cardiometabolic Health, Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, and Stroke Council. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation 135: e96–e121, 2017. doi: 10.1161/CIR.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu S, Surampudi P, Rosharavan B, Chondronikola M. Intermittent fasting as a nutrition approach against obesity and metabolic disease. Curr Opin Clin Nutr Metab Care 23: 387–394, 2020. doi: 10.1097/MCO.0000000000000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoddy KK, Marlatt KL, Cetinkaya H, Ravussin E. Intermittent fasting and metabolic health: from religious fast to time-restricted feeding. Obesity (Silver Spring) 28: S29–S37, 2020. doi: 10.1002/oby.22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirsh SP, Pons M, Joyal SV, Swick AG. Avoiding holiday seasonal weight gain with nutrient-supported intermittent energy restriction: a pilot study. J Nutr Sci 8: e11, 2019. doi: 10.1017/jns.2019.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Antoni R, Johnston KL, Collins AL, Robertson MD. Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br J Nutr 119: 507–516, 2018. doi: 10.1017/S0007114517003890. [DOI] [PubMed] [Google Scholar]
- 47. Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S, Wang R, Carlson OD, Egan JM, Mattson MP, Howell A. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr 110: 1534–1547, 2013. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 35: 714–727, 2011. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sundfør TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr Metab Cardiovasc Dis 28: 698–706, 2018. doi: 10.1016/j.numecd.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 50. Schübel R, Nattenmüller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, Schlett CL, von Stackelberg O, Johnson T, Nabers D, Kirsten R, Kratz M, Kauczor HU, Ulrich CM, Kaaks R, Kühn T. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr 108: 933–945, 2018. doi: 10.1093/ajcn/nqy196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 22: 86–99, 2015. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S, Cohen P, Morgan TE, Dorff T, Hong K, Michalsen A, Laviano A, Longo VD. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med 9: eaai8700, 2017. doi: 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gabel K, Cienfuegos S, Kalam F, Ezpeleta M, Varady KA. Time-restricted eating to improve cardiovascular health. Curr Atheroscler Rep 23: 22, 2021. doi: 10.1007/s11883-021-00922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20: 991–1005, 2014. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 14: 290, 2016. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 4: 345–353, 2018. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE, Shepherd JA, Weiss EJ. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med 180: 1491–1499, 2020. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kotarsky CJ, Johnson NR, Mahoney SJ, Mitchell SL, Schimek RL, Stastny SN, Hackney KJ. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep 9: e14868, 2021. doi: 10.14814/phy2.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jones R, Pabla P, Mallinson J, Nixon A, Taylor T, Bennett A, Tsintzas K. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am J Clin Nutr 112: 1015–1028, 2020. doi: 10.1093/ajcn/nqaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chow LS, Manoogian EN, Alvear A, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb S, Harindhanavudhi T, Nair KS, Panda S, Mashek DG. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring) 28: 860–869, 2020. doi: 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, Pahor M. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients 11: 1500, 2019. doi: 10.3390/nu11071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, Harry JR, VanDusseldorp TA, Kennedy DN, Cruz MR. Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr 110: 628–640, 2019. doi: 10.1093/ajcn/nqz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr Res 75: 32–43, 2020. doi: 10.1016/j.nutres.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 64. Kesztyüs D, Cermak P, Gulich M, Kesztyüs T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre-post design. Nutrients 11: 2854, 2019. doi: 10.3390/nu11122854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Parr EB, Devlin BL, Radford BE, Hawley JA. A delayed morning and earlier evening time-restricted feeding protocol for improving glycemic control and dietary adherence in men with overweight/obesity: a randomized controlled trial. Nutrients 12: 505, 2020. doi: 10.3390/nu12020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med 19: 148, 2021. doi: 10.1186/s12967-021-02817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stratton MT, Tinsley GM, Alesi MG, Hester GM, Olmos AA, Serafini PR, Modjeski AS, Mangine GT, King K, Savage SN, Webb AT, VanDusseldorp TA. Four weeks of time-restricted feeding combined with resistance training does not differentially influence measures of body composition, muscle performance, resting energy expenditure, and blood biomarkers. Nutrients 12: 1126, 2020. doi: 10.3390/nu12041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brady AJ, Langton HM, Mulligan M, Egan B. Effects of 8 wk of 16:8 time-restricted eating in male middle- and long-distance runners. Med Sci Sports Exerc 53: 633–642, 2021. doi: 10.1249/MSS.0000000000002488. [DOI] [PubMed] [Google Scholar]
- 69. Correia JM, Santos I, Pezarat-Correia P, Minderico C, Schoenfeld BJ, Mendonca GV. Effects of time-restricted feeding on supramaximal exercise performance and body composition: a randomized and counterbalanced crossover study in healthy men. Int J Environ Res Public Health 18: 7227, 2021. doi: 10.3390/ijerph18147227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zeb F, Wu X, Chen L, Fatima S, Haq IU, Chen A, Majeed F, Feng Q, Li M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr 123: 1216–1226, 2020. doi: 10.1017/S0007114519003428. [DOI] [PubMed] [Google Scholar]
- 71. Martens CR, Rossman MJ, Mazzo MR, Jankowski LR, Nagy EE, Denman BA, Richey JJ, Johnson SA, Ziemba BP, Wang Y, Peterson CM, Chonchol M, Seals DR. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience 42: 667–686, 2020. doi: 10.1007/s11357-020-00156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim H, Jang BJ, Jung AR, Kim J, Ju HJ, Kim YI. The impact of time-restricted diet on sleep and metabolism in obese volunteers. Medicina (Kaunas) 56: 540, 2020. doi: 10.3390/medicina56100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moro T, Tinsley G, Longo G, Grigoletto D, Bianco A, Ferraris C, Guglielmetti M, Veneto A, Tagliabue A, Marcolin G, Paoli A. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: a randomized controlled trial. J Int Soc Sports Nutr 17: 65, 2020. doi: 10.1186/s12970-020-00396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schroder JD, Falqueto H, Mânica A, Zanini D, de Oliveira T, de Sá CA, Cardoso AM, Manfredi LH. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Transl Med 19: 3, 2021. doi: 10.1186/s12967-020-02687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Przulj D, Ladmore D, Smith KM, Phillips-Waller A, Hajek P. Time restricted eating as a weight loss intervention in adults with obesity. PLoS One 16: e0246186, 2021. doi: 10.1371/journal.pone.0246186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Domaszewski P, Konieczny M, Pakosz P, Baczkowicz D, Sadowska-Krepa E. Effect of a six-week intermittent fasting intervention program on the composition of the human body in women over 60 years of age. Int J Environ Res Public Health 17: 4138, 2020. doi: 10.3390/ijerph17114138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Isenmann E, Dissemond J, Geisler S. The effects of a macronutrient-based diet and time-restricted feeding (16:8) on body composition in physically active individuals—a 14-week randomised controlled trial. Nutrients 13: 3122, 2021. doi: 10.3390/nu13093122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tovar AP, Richardson CE, Keim NL, Van Loan MD, Davis BA, Casazza GA. Four weeks of 16/8 time restrictive feeding in endurance trained male runners decreases fat mass, without affecting exercise performance. Nutrients 13: 2941, 2021. doi: 10.3390/nu13092941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Moro T, Tinsley G, Pacelli FQ, Marcolin G, Bianco A, Paoli A. Twelve months of time-restricted eating and resistance training improve inflammatory markers and cardiometabolic risk factors. Med Sci Sports Exerc 53: 2577–2585, 2021. doi: 10.1249/MSS.0000000000002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lin YJ, Wang YT, Chan LC, Chu NF. Effect of time-restricted feeding on body composition and cardio-metabolic risk in middle-aged women in Taiwan. Nutrition 93: 111504, 2022. doi: 10.1016/j.nut.2021.111504. [DOI] [PubMed] [Google Scholar]
- 81. Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, Chen RQ, Chen ZY. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol 19: 219, 2019. doi: 10.1186/s12876-019-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park SJ, Yang JW, Song YJ. The effect of four weeks dietary intervention with 8-hour time-restricted eating on body composition and cardiometabolic risk factors in young adults. Nutrients 13: 2164, 2021. doi: 10.3390/nu13072164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, Guo D, Lin J, Xu B, Li C, He H, He J, Liu S, Shi L, Xue Y, Zhang H. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med 386: 1495–1504, 2022. doi: 10.1056/NEJMoa2114833. [DOI] [PubMed] [Google Scholar]
- 84. Xie Z, Sun Y, Ye Y, Hu D, Zhang H, He Z, Zhao H, Yang H, Mao Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat Commun 13: 1003, 2022. doi: 10.1038/s41467-022-28662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, Varady KA. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab 32: 366–378.e3, 2020. doi: 10.1016/j.cmet.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci 17: 200–207, 2017. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- 87. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27: 1212–1221.e3, 2018. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hutchison AT, Regmi P, Manoogian EN, Fleischer JG, Wittert GA, Panda S, Heilbronn LK. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring) 27: 724–732, 2019. doi: 10.1002/oby.22449. [DOI] [PubMed] [Google Scholar]
- 89. Parr EB, Devlin BL, Lim KH, Moresi LN, Geils C, Brennan L, Hawley JA. Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study. Nutrients 12: 3228, 2020. doi: 10.3390/nu12113228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wilkinson MJ, Manoogian EN, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Taub PR. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 31: 92–104.e5, 2020. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Peeke PM, Greenway FL, Billes SK, Zhang D, Fujioka K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial. Nutr Diabetes 11: 6, 2021. doi: 10.1038/s41387-021-00149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Prasad M, Fine K, Gee A, Nair N, Popp CJ, Cheng B, Manoogian EN, Panda S, Laferrère B. A smartphone intervention to promote time restricted eating reduces body weight and blood pressure in adults with overweight and obesity: a pilot study. Nutrients 13: 2148, 2021. doi: 10.3390/nu13072148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: a randomised controlled trial. Nutr Metab (Lond) 18: 88, 2021. doi: 10.1186/s12986-021-00613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao L, Hutchison AT, Liu B, Yates CL, Teong XT, Wittert GA, Thompson CH, Nguyen L, Au J, Manoogian EN, Le HD, Williams AE, Panda S, Banks S, Heilbronn LK. Time-restricted eating improves glycemic control and dampens energy-consuming pathways in human adipose tissue. Nutrition 96: 111583, 2022. doi: 10.1016/j.nut.2021.111583. [DOI] [PubMed] [Google Scholar]
- 95. Thomas EA, Zaman A, Sloggett KJ, Steinke S, Grau L, Catenacci VA, Cornier MA, Rynders CA. Early time-restricted eating compared with daily caloric restriction: randomized trial in adults with obesity. Obesity (Silver Spring) 30: 1027–1038, 2022. doi: 10.1002/oby.23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pureza IR, Melo IS, Macena ML, Praxedes DR, Vasconcelos LG, Silva-Júnior AE, Florêncio TM, Bueno NB. Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: randomized trial. Nutrition 77: 110796, 2020. doi: 10.1016/j.nut.2020.110796. [DOI] [PubMed] [Google Scholar]
- 97. de Oliveira Maranhão Pureza IR, da Silva Junior AE, Silva Praxedes DR, Lessa Vasconcelos LG, de Lima Macena M, Vieira de Melo IS, de Menezes Toledo Florêncio TM, Bueno NB. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: a 12-month randomized clinical trial. Clin Nutr 40: 759–766, 2021. doi: 10.1016/j.clnu.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 98. Phillips NE, Mareschal J, Schwab N, Manoogian EN, Borloz S, Ostinelli G, Gauthier-Jaques A, Umwali S, Gonzalez Rodriguez E, Aeberli D, Hans D, Panda S, Rodondi N, Naef F, Collet TH. The effects of time-restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: a pragmatic randomised controlled trial in community-based adults. Nutrients 13: 1042, 2021. doi: 10.3390/nu13031042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kesztyüs D, Vorwieger E, Schönsteiner D, Gulich M, Kesztyüs T. Applicability of time-restricted eating for the prevention of lifestyle-dependent diseases in a working population: results of a pilot study in a pre-post design. Ger Med Sci 19: Doc04, 2021. doi: 10.3205/000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 52: 1738–1748, 2003. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 101. Singhal P, Caumo A, Carey PE, Cobelli C, Taylor R. Regulation of endogenous glucose production after a mixed meal in type 2 diabetes. Am J Physiol Endocrinol Metab 283: E275–E283, 2002. doi: 10.1152/ajpendo.00424.2001. [DOI] [PubMed] [Google Scholar]
- 102. Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 18: 716–738, 1997. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 103. Frayn KN, Evans RD. Human Metabolism: a Regulatory Perspective. Hoboken, NJ: Wiley-Blackwell, 2019. [Google Scholar]
- 104. Ruge T, Hodson L, Cheeseman J, Dennis AL, Fielding BA, Humphreys SM, Frayn KN, Karpe F. Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab 94: 1781–1788, 2009. doi: 10.1210/jc.2008-2090. [DOI] [PubMed] [Google Scholar]
- 105. Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, Cobelli C. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes 54: 3265–3273, 2005. doi: 10.2337/diabetes.54.11.3265. [DOI] [PubMed] [Google Scholar]
- 106. Burke LM, Winter JA, Cameron-Smith D, Enslen M, Farnfield M, Decombaz J. Effect of intake of different dietary protein sources on plasma amino acid profiles at rest and after exercise. Int J Sport Nutr Exerc Metab 22: 452–462, 2012. doi: 10.1123/ijsnem.22.6.452. [DOI] [PubMed] [Google Scholar]
- 107. Yoshii N, Sato K, Ogasawara R, Nishimura Y, Shinohara Y, Fujita S. Effect of mixed meal and leucine intake on plasma amino acid concentrations in young men. Nutrients 10: 1543, 2018. doi: 10.3390/nu10101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lambert JE, Parks EJ. Postprandial metabolism of meal triglyceride in humans. Biochim Biophys Acta 1821: 721–726, 2012. doi: 10.1016/j.bbalip.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Evans K, Kuusela PJ, Cruz ML, Wilhelmova I, Fielding BA, Frayn KN. Rapid chylomicron appearance following sequential meals: effects of second meal composition. Br J Nutr 79: 425–429, 1998. doi: 10.1079/bjn19980072. [DOI] [PubMed] [Google Scholar]
- 110. Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, Keim NL, Havel PJ. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 96: E1596–E1605, 2011. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jackson KG, Abraham EC, Smith AM, Murray P, O’Malley B, Williams CM, Minihane AM. Impact of age and menopausal status on the postprandial triacylglycerol response in healthy women. Atherosclerosis 208: 246–252, 2010. doi: 10.1016/j.atherosclerosis.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 112. Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav 106: 387–393, 2012. doi: 10.1016/j.physbeh.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gutniak M, Grill V, Efendić S. Effect of composition of mixed meals–low- versus high-carbohydrate content–on insulin, glucagon, and somatostatin release in healthy humans and in patients with NIDDM. Diabetes Care 9: 244–249, 1986. doi: 10.2337/diacare.9.3.244. [DOI] [PubMed] [Google Scholar]
- 114. Geary N. Effects of glucagon, insulin, amylin and CGRP on feeding. Neuropeptides 33: 400–405, 1999. doi: 10.1054/npep.1999.0046. [DOI] [PubMed] [Google Scholar]
- 115. Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14: 272–279, 2011. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 116. Dent P, Lavoinne A, Nakielny S, Caudwell FB, Watt P, Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature 348: 302–308, 1990. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- 117. Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care 35: 1316–1321, 2012. doi: 10.2337/dc11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Edgerton DS, Ramnanan CJ, Grueter CA, Johnson KM, Lautz M, Neal DW, Williams PE, Cherrington AD. Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 58: 2766–2775, 2009. doi: 10.2337/db09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bieberdorf FA, Chernick SS, Scow RO. Effect of insulin and acute diabetes on plasma FFA and ketone bodies in the fasting rat. J Clin Invest 49: 1685–1693, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Topping DL, Mayes PA. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J 126: 295–311, 1972. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Smith GI, Atherton P, Reeds DN, Mohammed BS, Jaffery H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol (1985) 107: 1308–1315, 2009. doi: 10.1152/japplphysiol.00348.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Scherer T, O’Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab 13: 183–194, 2011. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yost TJ, Jensen DR, Haugen BR, Eckel RH. Effect of dietary macronutrient composition on tissue-specific lipoprotein lipase activity and insulin action in normal-weight subjects. Am J Clin Nutr 68: 296–302, 1998. doi: 10.1093/ajcn/68.2.296. [DOI] [PubMed] [Google Scholar]
- 124. Holst JJ, Christensen M, Lund A, de Heer J, Svendsen B, Kielgast U, Knop FK. Regulation of glucagon secretion by incretins. Diabetes Obes Metab 13, Suppl 1: 89–94, 2011. doi: 10.1111/j.1463-1326.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- 125. Sherr J, Tsalikian E, Fox L, Buckingham B, Weinzimer S, Tamborlane WV, White NH, Arbelaez AM, Kollman C, Ruedy KJ, Cheng P, Beck RW; Diabetes Research in Children Network (DirecNet). Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis. Diabetes Care 37: 1741–1744, 2014. doi: 10.2337/dc13-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA 107: 16009–16012, 2010. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cahill GF Jr. Starvation in man. N Engl J Med 282: 668–675, 1970. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 128. Ang T, Bruce CR, Kowalski GM. Postprandial aminogenic insulin and glucagon secretion can stimulate glucose flux in humans. Diabetes 68: 939–946, 2019. doi: 10.2337/db18-1138. [DOI] [PubMed] [Google Scholar]
- 129. Morgan LM, Aspostolakou F, Wright J, Gama R. Diurnal variations in peripheral insulin resistance and plasma non-esterified fatty acid concentrations: a possible link? Ann Clin Biochem 36: 447–450, 1999. doi: 10.1177/000456329903600407. [DOI] [PubMed] [Google Scholar]
- 130. Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes 41: 750–759, 1992. doi: 10.2337/diab.41.6.750. [DOI] [PubMed] [Google Scholar]
- 131. Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61: 2691–2700, 2012. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jarrett RJ, Baker IA, Keen H, Oakley NW. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon, and evening. Br Med J 1: 199–201, 1972. doi: 10.1136/bmj.1.5794.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zimmet PZ, Wall JR, Rome R, Stimmler L, Jarrett RJ. Diurnal variation in glucose tolerance: associated changes in plasma insulin, growth hormone, and non-esterified fatty acids. Br Med J 1: 485–488, 1974. doi: 10.1136/bmj.1.5906.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lindgren O, Mari A, Deacon CF, Carr RD, Winzell MS, Vikman J, Ahrén B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J Clin Endocrinol Metab 94: 2887–2892, 2009. doi: 10.1210/jc.2009-0366. [DOI] [PubMed] [Google Scholar]
- 135. Yoshino J, Almeda-Valdes P, Patterson BW, Okunade AL, Imai S, Mittendorfer B, Klein S. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J Clin Endocrinol Metab 99: E1666–E1670, 2014. doi: 10.1210/jc.2014-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 88: 934–942, 1991. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sonnier T, Rood J, Gimble JM, Peterson CM. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J Diabetes Complications 28: 836–843, 2014. doi: 10.1016/j.jdiacomp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 138. Jovanovic A, Gerrard J, Taylor R. The second-meal phenomenon in type 2 diabetes. Diabetes Care 32: 1199–1201, 2009. doi: 10.2337/dc08-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jovanovic A, Leverton E, Solanky B, Ravikumar B, Snaar JE, Morris PG, Taylor R. The second-meal phenomenon is associated with enhanced muscle glycogen storage in humans. Clin Sci (Lond) 117: 119–127, 2009. doi: 10.1042/CS20080542. [DOI] [PubMed] [Google Scholar]
- 140. Ando T, Nakae S, Usui C, Yoshimura E, Nishi N, Takimoto H, Tanaka S. Effect of diurnal variations in the carbohydrate and fat composition of meals on postprandial glycemic response in healthy adults: a novel insight for the second-meal phenomenon. Am J Clin Nutr 108: 332–342, 2018. doi: 10.1093/ajcn/nqy086. [DOI] [PubMed] [Google Scholar]
- 141. Hayner NS, Kjelsberg MO, Epstein FH, Francis T Jr.. Carbohydrate tolerance and diabetes in a total community, Tecumseh, Michigan. 1. effects of age, sex, and test conditions on one-hour glucose tolerance in adults. Diabetes 14: 413–423, 1965. doi: 10.2337/diab.14.7.413. [DOI] [PubMed] [Google Scholar]
- 142. Yalow RS, Goldsmith SJ, Berson SA. Influence of physiologic fluctuations in plasma growth hormone on glucose tolerance. Diabetes 18: 402–408, 1969. doi: 10.2337/diab.18.6.402. [DOI] [PubMed] [Google Scholar]
- 143. Carey PE, Halliday J, Snaar JE, Morris PG, Taylor R. Direct assessment of muscle glycogen storage after mixed meals in normal and type 2 diabetic subjects. Am J Physiol Endocrinol Metab 284: E688–E694, 2003. doi: 10.1152/ajpendo.00471.2002. [DOI] [PubMed] [Google Scholar]
- 144. Lee SH, Tura A, Mari A, Ko SH, Kwon HS, Song KH, Yoon KH, Lee KW, Ahn YB. Potentiation of the early-phase insulin response by a prior meal contributes to the second-meal phenomenon in type 2 diabetes. Am J Physiol Endocrinol Metab 301: E984–E990, 2011. doi: 10.1152/ajpendo.00244.2011. [DOI] [PubMed] [Google Scholar]
- 145. Collier GR, Wolever TM, Jenkins DJ. Concurrent ingestion of fat and reduction in starch content impairs carbohydrate tolerance to subsequent meals. Am J Clin Nutr 45: 963–969, 1987. doi: 10.1093/ajcn/45.5.963. [DOI] [PubMed] [Google Scholar]
- 146. Wolever TM, Bentum-Williams A, Jenkins DJ. Physiological modulation of plasma free fatty acid concentrations by diet. Metabolic implications in nondiabetic subjects. Diabetes Care 18: 962–970, 1995. doi: 10.2337/diacare.18.7.962. [DOI] [PubMed] [Google Scholar]
- 147. Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr 48: 1041–1047, 1988. doi: 10.1093/ajcn/48.4.1041. [DOI] [PubMed] [Google Scholar]
- 148. Ahmed M, Gannon MC, Nuttall FQ. Postprandial plasma glucose, insulin, glucagon and triglyceride responses to a standard diet in normal subjects. Diabetologia 12: 61–67, 1976. doi: 10.1007/BF01221966. [DOI] [PubMed] [Google Scholar]
- 149. Service FJ, Hall LD, Westland RE, O’Brien PC, Go VL, Haymond MW, Rizza RA. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia 25: 316–321, 1983. doi: 10.1007/BF00253193. [DOI] [PubMed] [Google Scholar]
- 150. Leiter LA, Marliss EB. Survival during fasting may depend on fat as well as protein stores. JAMA 248: 2306–2307, 1982. doi: 10.1001/jama.1982.03330180066037. [DOI] [PubMed] [Google Scholar]
- 151. Stewart WK, Fleming LW. Features of a successful therapeutic fast of 382 days’ duration. Postgrad Med J 49: 203–209, 1973. doi: 10.1136/pgmj.49.569.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Klein S, Sakurai Y, Romijn JA, Carroll RM. Progressive alterations in lipid and glucose metabolism during short-term fasting in young adult men. Am J Physiol Endocrinol Metab 265: E801–E806, 1993. doi: 10.1152/ajpendo.1993.265.5.E801. [DOI] [PubMed] [Google Scholar]
- 153. Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science 254: 573–576, 1991. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 154. Nilsson LH, Hultman E. Liver glycogen in man—the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest 32: 325–330, 1973. doi: 10.3109/00365517309084355. [DOI] [PubMed] [Google Scholar]
- 155. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36: 587–597, 2013. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Balasse EO. Kinetics of ketone body metabolism in fasting humans. Metabolism 28: 41–50, 1979. doi: 10.1016/0026-0495(79)90166-5. [DOI] [PubMed] [Google Scholar]
- 157. Aoki TT. Metabolic adaptations to starvation, semistarvation, and carbohydrate restriction. Prog Clin Biol Res 67: 161–177, 1981. [PubMed] [Google Scholar]
- 158. Klein S, Wolfe RR. Carbohydrate restriction regulates the adaptive response to fasting. Am J Physiol Endocrinol Metab 262: E631–E636, 1992. doi: 10.1152/ajpendo.1992.262.5.E631. [DOI] [PubMed] [Google Scholar]
- 159. López-Otín C, Kroemer G. Hallmarks of health. Cell 184: 33–63, 2021. doi: 10.1016/j.cell.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 160. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health 25: 85–93, 2001. [PMC free article] [PubMed] [Google Scholar]
- 162. Kidd PB, Young MW, Siggia ED. Temperature compensation and temperature sensation in the circadian clock. Proc Natl Acad Sci USA 112: E6284–E6292, 2015. doi: 10.1073/pnas.1511215112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev 90: 1063–1102, 2010. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 164. Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 165. Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA 108: 18790–18795, 2011. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Dodd AN, Belbin FE, Frank A, Webb AA. Interactions between circadian clocks and photosynthesis for the temporal and spatial coordination of metabolism. Front Plant Sci 6: 245, 2015. doi: 10.3389/fpls.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science 241: 1225–1227, 1988. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- 168. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549, 2010. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 169. Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, Sinturel F, Gosselin P, Gerber A, Fleury-Olela F, Rando G, Demarque M, Franken P. Clock-talk: interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb Symp Quant Biol 80: 223–232, 2015. doi: 10.1101/sqb.2015.80.027490. [DOI] [PubMed] [Google Scholar]
- 170. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA 106: 21453–21458, 2009. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, Maywood ES, Stangherlin A, Chesham JE, Hayter EA, Rosenbrier-Ribeiro L, Newham P, Clevers H, Bechtold DA, O’Neill JS. Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 177: 896–909.e20, 2019. doi: 10.1016/j.cell.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Manella G, Sabath E, Aviram R, Dandavate V, Ezagouri S, Golik M, Adamovich Y, Asher G. The liver-clock coordinates rhythmicity of peripheral tissues in response to feeding. Nat Metab 3: 829–842, 2021. doi: 10.1038/s42255-021-00395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hughes ME, Abruzzi KC, Allada R, Anafi R, Arpat AB, Asher G, et al. Guidelines for genome-scale analysis of biological rhythms. J Biol Rhythms 32: 380–393, 2017. doi: 10.1177/0748730417728663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wu G, Anafi RC, Hughes ME, Kornacker K, Hogenesch JB. MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics 32: 3351–3353, 2016. doi: 10.1093/bioinformatics/btw405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Dunlap JC. Molecular bases for circadian clocks. Cell 96: 271–290, 1999. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 177. Cox KH, Takahashi JS. Introduction to the clock system. Adv Exp Med Biol 1344: 3–20, 2021. doi: 10.1007/978-3-030-81147-1_1. [DOI] [PubMed] [Google Scholar]
- 178. Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725, 1994. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell 89: 641–653, 1997. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017, 2000. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18: 164–179, 2017. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107: 855–867, 2001. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 183. Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8: 139–148, 2007. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 184. Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 485: 123–127, 2012. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, Panda S. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359: eaao0318, 2018. doi: 10.1126/science.aao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111: 16219–16224, 2014. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Saran AR, Dave S, Zarrinpar A. Circadian rhythms in the pathogenesis and treatment of fatty liver disease. Gastroenterology 158: 1948–1966.e1, 2020. doi: 10.1053/j.gastro.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326: 437–440, 2009. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Li MD, Ruan HB, Hughes ME, Lee JS, Singh JP, Jones SP, Nitabach MN, Yang X. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab 17: 303–310, 2013. doi: 10.1016/j.cmet.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kaasik K, Kivimäe S, Allen JJ, Chalkley RJ, Huang Y, Baer K, Kissel H, Burlingame AL, Shokat KM, Ptáček LJ, Fu YH. Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab 17: 291–302, 2013. doi: 10.1016/j.cmet.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Chen R, Schirmer A, Lee Y, Lee H, Kumar V, Yoo SH, Takahashi JS, Lee C. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell 36: 417–430, 2009. doi: 10.1016/j.molcel.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ong Q, Han W, Yang X. O-GlcNAc as an integrator of signaling pathways. Front Endocrinol (Lausanne) 9: 599, 2018. doi: 10.3389/fendo.2018.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc Natl Acad Sci USA 105: 20746–20751, 2008. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem 280: 29397–29402, 2005. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- 195. Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. J Biol Chem 280: 31714–31721, 2005. doi: 10.1074/jbc.M506225200. [DOI] [PubMed] [Google Scholar]
- 196. Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One 5: e8561, 2010. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Spengler ML, Kuropatwinski KK, Schumer M, Antoch MP. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle 8: 4138–4146, 2009. doi: 10.4161/cc.8.24.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science 311: 1002–1005, 2006. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 199. Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Güttler T, Davis F, Asara JM, Sahin M. The circadian protein BMAL1 regulates translation in response to S6K1-mediated phosphorylation. Cell 161: 1138–1151, 2015. doi: 10.1016/j.cell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Khapre RV, Patel SA, Kondratova AA, Chaudhary A, Velingkaar N, Antoch MP, Kondratov RV. Metabolic clock generates nutrient anticipation rhythms in mTOR signaling. Aging (Albany NY) 6: 675–689, 2014. doi: 10.18632/aging.100686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Wu R, Dang F, Li P, Wang P, Xu Q, Liu Z, Li Y, Wu Y, Chen Y, Liu Y. The circadian protein Period2 suppresses mTORC1 activity via recruiting Tsc1 to mTORC1 complex. Cell Metab 29: 653–667.e6, 2019. doi: 10.1016/j.cmet.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 202. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 98: 2133–2223, 2018. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Dang F, Sun X, Ma X, Wu R, Zhang D, Chen Y, Xu Q, Wu Y, Liu Y. Insulin post-transcriptionally modulates Bmal1 protein to affect the hepatic circadian clock. Nat Commun 7: 12696, 2016. doi: 10.1038/ncomms12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Luciano AK, Zhou W, Santana JM, Kyriakides C, Velazquez H, Sessa WC. CLOCK phosphorylation by AKT regulates its nuclear accumulation and circadian gene expression in peripheral tissues. J Biol Chem 293: 9126–9136, 2018. doi: 10.1074/jbc.RA117.000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab 20: 325–331, 2009. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev 93: 107–135, 2013. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324: 654–657, 2009. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 209. Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab 13: 125–137, 2011. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 210. Zhou B, Zhang Y, Zhang F, Xia Y, Liu J, Huang R, Wang Y, Hu Y, Wu J, Dai C, Wang H, Tu Y, Peng X, Wang Y, Zhai Q. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 59: 2196–2206, 2014. doi: 10.1002/hep.26992. [DOI] [PubMed] [Google Scholar]
- 211. Levine DC, Hong H, Weidemann BJ, Ramsey KM, Affinati AH, Schmidt MS, Cedernaes J, Omura C, Braun R, Lee C, Brenner C, Peek CB, Bass J. NAD+ controls circadian reprogramming through PER2 nuclear translocation to counter aging. Mol Cell 78: 835–849.e7, 2020. doi: 10.1016/j.molcel.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci USA 99: 7728–7733, 2002. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 16: 1152–1156, 2010. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 62: 2195–2203, 2013. doi: 10.2337/db12-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466: 627–631, 2010. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Delezie J, Dumont S, Dardente H, Oudart H, Gréchez-Cassiau A, Klosen P, Teboul M, Delaunay F, Pévet P, Challet E. The nuclear receptor REV-ERBalpha is required for the daily balance of carbohydrate and lipid metabolism. FASEB J 26: 3321–3335, 2012. doi: 10.1096/fj.12-208751. [DOI] [PubMed] [Google Scholar]
- 218. Adlanmerini M, Krusen BM, Nguyen HC, Teng CW, Woodie LN, Tackenberg MC, Geisler CE, Gaisinsky J, Peed LC, Carpenter BJ, Hayes MR, Lazar MA. REV-ERB nuclear receptors in the suprachiasmatic nucleus control circadian period and restrict diet-induced obesity. Sci Adv 7: eabh2007, 2021. doi: 10.1126/sciadv.abh2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zani F, Breasson L, Becattini B, Vukolic A, Montani JP, Albrecht U, Provenzani A, Ripperger JA, Solinas G. PER2 promotes glucose storage to liver glycogen during feeding and acute fasting by inducing Gys2 PTG and G L expression. Mol Metab 2: 292–305, 2013. doi: 10.1016/j.molmet.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Feillet CA, Ripperger JA, Magnone MC, Dulloo A, Albrecht U, Challet E. Lack of food anticipation in Per2 mutant mice. Curr Biol 16: 2016–2022, 2006. doi: 10.1016/j.cub.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 221. Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab 19: 319–330, 2014. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Barclay JL, Shostak A, Leliavski A, Tsang AH, Jöhren O, Müller-Fielitz H, Landgraf D, Naujokat N, van der Horst GT, Oster H. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am J Physiol Endocrinol Metab 304: E1053–E1063, 2013. doi: 10.1152/ajpendo.00512.2012. [DOI] [PubMed] [Google Scholar]
- 223. Griebel G, Ravinet-Trillou C, Beeské S, Avenet P, Pichat P. Mice deficient in cryptochrome 1 (Cry1-/-) exhibit resistance to obesity induced by a high-fat diet. Front Endocrinol (Lausanne) 5: 49, 2014. doi: 10.3389/fendo.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Cedernaes J, Huang W, Ramsey KM, Waldeck N, Cheng L, Marcheva B, Omura C, Kobayashi Y, Peek CB, Levine DC, Dhir R, Awatramani R, Bradfield CA, Wang XA, Takahashi JS, Mokadem M, Ahima RS, Bass J. Transcriptional basis for rhythmic control of hunger and metabolism within the AgRP neuron. Cell Metab 29: 1078–1091.e5, 2019. doi: 10.1016/j.cmet.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kolbe I, Leinweber B, Brandenburger M, Oster H. Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol Metab 30: 140–151, 2019. doi: 10.1016/j.molmet.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Yang G, Zhang J, Jiang T, Monslow J, Tang SY, Todd L, Puré E, Chen L, FitzGerald GA. Bmal1 deletion in myeloid cells attenuates atherosclerotic lesion development and restrains abdominal aortic aneurysm formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol 40: 1523–1532, 2020. doi: 10.1161/ATVBAHA.120.314318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868–1873, 2006. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab 29: 303–319.e4, 2019. doi: 10.1016/j.cmet.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 18: 171–195, 1994. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 230. Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6: 269–278, 2001. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 231. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 232. Fougeray T, Polizzi A, Régnier M, Fougerat A, Ellero-Simatos S, Lippi Y, Smati S, Lasserre F, Tramunt B, Huillet M, Dopavogui L, Salvi J, Nédélec E, Gigot V, Smith L, Naylies C, Sommer C, Haas JT, Wahli W, Duez H, Gourdy P, Gamet-Payrastre L, Benani A, Burnol AF, Loiseau N, Postic C, Montagner A, Guillou H. The hepatocyte insulin receptor is required to program the liver clock and rhythmic gene expression. Cell Rep 39: 110674, 2022. doi: 10.1016/j.celrep.2022.110674. [DOI] [PubMed] [Google Scholar]
- 233. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15: 848–860, 2012. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 235. Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 236. Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2: e377, 2004. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Saini C, Liani A, Curie T, Gos P, Kreppel F, Emmenegger Y, Bonacina L, Wolf JP, Poget YA, Franken P, Schibler U. Real-time recording of circadian liver gene expression in freely moving mice reveals the phase-setting behavior of hepatocyte clocks. Genes Dev 27: 1526–1536, 2013. doi: 10.1101/gad.221374.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Greenwell BJ, Trott AJ, Beytebiere JR, Pao S, Bosley A, Beach E, Finegan P, Hernandez C, Menet JS. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice. Cell Rep 27: 649–657.e5, 2019. doi: 10.1016/j.celrep.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 239. Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284: 2177–2181, 1999. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 240. Wright KP Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA 98: 14027–14032, 2001. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms 17: 181–193, 2002. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 242. Klerman H, St Hilaire MA, Kronauer RE, Gooley JJ, Gronfier C, Hull JT, Lockley SW, Santhi N, Wang W, Klerman EB. Analysis method and experimental conditions affect computed circadian phase from melatonin data. PLoS One 7: e33836, 2012. doi: 10.1371/journal.pone.0033836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 12: 457–466, 1997. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 244. Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms 20: 168–177, 2005. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Wright KP Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol 23: 1554–1558, 2013. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab 32: 266–284, 1971. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- 247. Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab 33: 14–22, 1971. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 248. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 11: 1234, 2019. doi: 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wehrens SM, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. Meal timing regulates the human circadian system. Curr Biol 27: 1768–1775.e3, 2017. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Allison KC, Hopkins CM, Ruggieri M, Spaeth AM, Ahima RS, Zhang Z, Taylor DM, Goel N. Prolonged, controlled daytime versus delayed eating impacts weight and metabolism. Curr Biol 31: 650–657.e3, 2021. doi: 10.1016/j.cub.2020.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Resuehr D, Wu G, Johnson RL Jr, Young ME, Hogenesch JB, Gamble KL. Shift work disrupts circadian regulation of the transcriptome in hospital nurses. J Biol Rhythms 34: 167–177, 2019. doi: 10.1177/0748730419826694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Depner CM, Melanson EL, McHill AW, Wright KP Jr.. Mistimed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc Natl Acad Sci USA 115: E5390–E5399, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev 30: 11–24, 2016. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 254. Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 58: 747–752, 2001. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Monk TH, Buysse DJ. Exposure to shift work as a risk factor for diabetes. J Biol Rhythms 28: 356–359, 2013. doi: 10.1177/0748730413506557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, Saxena R, Scheer F. Night shift work, genetic risk, and type 2 diabetes in the UK Biobank. Diabetes Care 41: 762–769, 2018. doi: 10.2337/dc17-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lee Y, Seo E, Mun E, Lee W. A longitudinal study of working hours and chronic kidney disease in healthy workers: the Kangbuk Samsung Health Study. J Occup Health 63: e12266, 2021. doi: 10.1002/1348-9585.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Torquati L, Mielke GI, Brown WJ, Kolbe-Alexander T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand J Work Environ Health 44: 229–238, 2018. doi: 10.5271/sjweh.3700. [DOI] [PubMed] [Google Scholar]
- 259. De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol 38: 848–854, 2009. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 260. Peñalvo JL, Mertens E, Muñoz-Cabrejas A, León-Latre M, Jarauta E, Laclaustra M, Ordovas JM, Casasnovas JA, Uzhova I, Moreno-Franco B. Work shift, lifestyle factors, and subclinical atherosclerosis in Spanish male workers: a mediation analysis. Nutrients 13: 1077, 2021. doi: 10.3390/nu13041077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Jermendy G, Nádas J, Hegyi I, Vasas I, Hidvégi T. Assessment of cardiometabolic risk among shift workers in Hungary. Health Qual Life Outcomes 10: 18, 2012. doi: 10.1186/1477-7525-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Rivera AS, Akanbi M, O’Dwyer LC, McHugh M. Shift work and long work hours and their association with chronic health conditions: a systematic review of systematic reviews with meta-analyses. PLoS One 15: e0231037, 2020. doi: 10.1371/journal.pone.0231037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Broussard JL, Reynolds AC, Depner CM, Ferguson SA, Dawson D, Wright KP. Circadian rhythms versus daily patterns in human physiology and behavior. In: Biological Timekeeping: Clocks, Rhythms and Behaviour, edited by Kumar V. New Delhi: Springer, 2017, p. 279–295. [Google Scholar]
- 264. Duffy JF, Cain SW, Chang AM, Phillips AJ, Münch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP Jr, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA 108: 15602–15608, 2011. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FA. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA 112: E2225–E2234, 2015. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106: 4453–4458, 2009. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 4: 129ra143, 2012. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Koritala BS, Porter KI, Arshad OA, Gajula RP, Mitchell HD, Arman T, Manjanatha MG, Teeguarden J, Van Dongen HP, McDermott JE, Gaddameedhi S. Night shift schedule causes circadian dysregulation of DNA repair genes and elevated DNA damage in humans. J Pineal Res 70: e12726, 2021. doi: 10.1111/jpi.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Grant CL, Coates AM, Dorrian J, Kennaway DJ, Wittert GA, Heilbronn LK, Pajcin M, Della Vedova C, Gupta CC, Banks S. Timing of food intake during simulated night shift impacts glucose metabolism: a controlled study. Chronobiol Int 34: 1003–1013, 2017. doi: 10.1080/07420528.2017.1335318. [DOI] [PubMed] [Google Scholar]
- 270. Chellappa SL, Qian J, Vujovic N, Morris CJ, Nedeltcheva A, Nguyen H, Rahman N, Heng SW, Kelly L, Kerlin-Monteiro K, Srivastav S, Wang W, Aeschbach D, Czeisler CA, Shea SA, Adler GK, Garaulet M, Scheer F. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci Adv 7: eabg9910, 2021. doi: 10.1126/sciadv.abg9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Centofanti S, Dorrian J, Hilditch C, Grant C, Coates A, Banks S. Eating on nightshift: a big vs. small snack impairs glucose response to breakfast. Neurobiol Sleep Circadian Rhythms 4: 44–48, 2018. doi: 10.1016/j.nbscr.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 151: 1019–1029, 2010. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 273. Brown MR, Sen SK, Mazzone A, Her TK, Xiong Y, Lee JH, Javeed N, Colwell CS, Rakshit K, LeBrasseur NK, Gaspar-Maia A, Ordog T, Matveyenko AV. Time-restricted feeding prevents deleterious metabolic effects of circadian disruption through epigenetic control of beta cell function. Sci Adv 7: eabg6856, 2021. doi: 10.1126/sciadv.abg6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17: 2100–2102, 2009. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Duncan MJ, Smith JT, Narbaiza J, Mueez F, Bustle LB, Qureshi S, Fieseler C, Legan SJ. Restricting feeding to the active phase in middle-aged mice attenuates adverse metabolic effects of a high-fat diet. Physiol Behav 167: 1–9, 2016. doi: 10.1016/j.physbeh.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 276. Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J 26: 3493–3502, 2012. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 277. Sundaram S, Yan L. Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr Res 36: 603–611, 2016. doi: 10.1016/j.nutres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 278. Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, Gonzalez R, Kueht M, McElfresh TA, Brewer RA, Chandler MP, Bray MS, Young ME. Influence of dark phase restricted high fat feeding on myocardial adaptation in mice. J Mol Cell Cardiol 55: 147–155, 2013. doi: 10.1016/j.yjmcc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab 20: 1006–1017, 2014. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Kentish SJ, Hatzinikolas G, Li H, Frisby CL, Wittert GA, Page AJ. Time-restricted feeding prevents ablation of diurnal rhythms in gastric vagal afferent mechanosensitivity observed in high-fat diet-induced obese mice. J Neurosci 38: 5088–5095, 2018. doi: 10.1523/JNEUROSCI.0052-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Aouichat S, Chayah M, Bouguerra-Aouichat S, Agil A. Time-restricted feeding improves body weight gain, lipid profiles, and atherogenic indices in cafeteria-diet-fed rats: role of browning of inguinal white adipose tissue. Nutrients 12: 2185, 2020. doi: 10.3390/nu12082185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. de Goede P, Hellings TP, Coopmans TV, Ritsema W, Kalsbeek A. After-effects of time-restricted feeding on whole-body metabolism and gene expression in four different peripheral tissues. Obesity (Silver Spring) 28, Suppl 1: S68–S80, 2020. doi: 10.1002/oby.22830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA 107: 18664–18669, 2010. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Laferrère B, Panda S. Calorie and time restriction in weight loss. N Engl J Med 386: 1572–1573, 2022. doi: 10.1056/NEJMe2202821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Rumpler WV, Baer DJ, Egan J, Mattson MP. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 56: 1729–1734, 2007. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Mattson MP. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr 85: 981–988, 2007. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Chung H, Chou W, Sears DD, Patterson RE, Webster NJ, Ellies LG. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism 65: 1743–1754, 2016. doi: 10.1016/j.metabol.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Boucsein A, Rizwan MZ, Tups A. Hypothalamic leptin sensitivity and health benefits of time-restricted feeding are dependent on the time of day in male mice. FASEB J 33: 12175–12187, 2019. doi: 10.1096/fj.201901004R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Chaix A, Deota S, Bhardwaj R, Lin T, Panda S. Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a Western high-fat high-sucrose diet in C57BL/6J mice. Cell Rep 36: 109543, 2021. doi: 10.1016/j.celrep.2021.109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Sorrell J, Yates E, Rivir M, Woods SC, Hogenesch JB, Perez-Tilve D. The central melanocortin system mediates the benefits of time-restricted feeding on energy balance. Physiol Behav 227: 113132, 2020. doi: 10.1016/j.physbeh.2020.113132. [DOI] [PubMed] [Google Scholar]
- 291. Oishi K, Hashimoto C. Short-term time-restricted feeding during the resting phase is sufficient to induce leptin resistance that contributes to development of obesity and metabolic disorders in mice. Chronobiol Int 35: 1576–1594, 2018. doi: 10.1080/07420528.2018.1496927. [DOI] [PubMed] [Google Scholar]
- 292. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity (Silver Spring) 27: 1244–1254, 2019. doi: 10.1002/oby.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Regmi P, Chaudhary R, Page AJ, Hutchison AT, Vincent AD, Liu B, Heilbronn L. Early or delayed time-restricted feeding prevents metabolic impact of obesity in mice. J Endocrinol 248: 75–86, 2021. doi: 10.1530/JOE-20-0404. [DOI] [PubMed] [Google Scholar]
- 294. Woodie LN, Luo Y, Wayne MJ, Graff EC, Ahmed B, O’Neill AM, Greene MW. Restricted feeding for 9 h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism 82: 1–13, 2018. doi: 10.1016/j.metabol.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 295. Abe T, Kazama R, Okauchi H, Oishi K. Food deprivation during active phase induces skeletal muscle atrophy via IGF-1 reduction in mice. Arch Biochem Biophys 677: 108160, 2019. doi: 10.1016/j.abb.2019.108160. [DOI] [PubMed] [Google Scholar]
- 296. Kelly KP, Ellacott KL, Chen H, McGuinness OP, Johnson CH. Time-optimized feeding is beneficial without enforced fasting. Open Biol 11: 210183, 2021. doi: 10.1098/rsob.210183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Yasumoto Y, Hashimoto C, Nakao R, Yamazaki H, Hiroyama H, Nemoto T, Yamamoto S, Sakurai M, Oike H, Wada N, Yoshida-Noro C, Oishi K. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 65: 714–727, 2016. doi: 10.1016/j.metabol.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 298. Acosta-Rodríguez VA, de Groot MH, Rijo-Ferreira F, Green CB, Takahashi JS. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab 26: 267–277.e2, 2017. doi: 10.1016/j.cmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Sherman H, Frumin I, Gutman R, Chapnik N, Lorentz A, Meylan J, Le Coutre J, Froy O. Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J Cell Mol Med 15: 2745–2759, 2011. doi: 10.1111/j.1582-4934.2010.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Lin S, Varady KA. Changes in body weight and metabolic risk during time restricted feeding in premenopausal versus postmenopausal women. Exp Gerontol 154: 111545, 2021. doi: 10.1016/j.exger.2021.111545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. McAllister MJ, Gonzalez AE, Waldman HS. Impact of time restricted feeding on markers of cardiometabolic health and oxidative stress in resistance-trained firefighters. J Strength Cond Res. 2020. doi: 10.1519/JSC.0000000000003860. [DOI] [PubMed] [Google Scholar]
- 302. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 303. Chaix A, Manoogian EN, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr 39: 291–315, 2019. doi: 10.1146/annurev-nutr-082018-124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Mehus AA, Rust B, Idso JP, Hanson B, Zeng H, Yan L, Bukowski MR, Picklo MJ. Time-restricted feeding mice a high-fat diet induces a unique lipidomic profile. J Nutr Biochem 88: 108531, 2021. doi: 10.1016/j.jnutbio.2020.108531. [DOI] [PubMed] [Google Scholar]
- 305. Dantas Machado AC, Brown SD, Lingaraju A, Sivaganesh V, Martino C, Chaix A, Zhao P, Chang MW, Richter RA, Saghatelian A, Saltiel AR, Knight R, Panda S, Zarrinpar A. Diet, feeding pattern and host genetics modulate diurnal dynamics of the ileal microbiome and transcriptome. Cell Rep 40: 111008, 2022. doi: 10.1016/j.celrep.2022.111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Hou T, Su W, Duncan MJ, Olga VA, Guo Z, Gong MC. Time-restricted feeding protects the blood pressure circadian rhythm in diabetic mice. Proc Natl Acad Sci USA 118: e2015873118, 2021. doi: 10.1073/pnas.2015873118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Pistrosch F, Reissmann E, Wildbrett J, Koehler C, Hanefeld M. Relationship between diurnal blood pressure variation and diurnal blood glucose levels in type 2 diabetic patients. Am J Hypertens 20: 541–545, 2007. doi: 10.1016/j.amjhyper.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 308. Ukkola O, Vasunta RL, Kesäniemi YA. Non-dipping pattern in ambulatory blood pressure monitoring is associated with metabolic abnormalities in a random sample of middle-aged subjects. Hypertens Res 32: 1022–1027, 2009. doi: 10.1038/hr.2009.137. [DOI] [PubMed] [Google Scholar]
- 309. Cote I, Toklu HZ, Green SM, Morgan D, Carter CS, Tümer N, Scarpace PJ. Limiting feeding to the active phase reduces blood pressure without the necessity of caloric reduction or fat mass loss. Am J Physiol Regul Integr Comp Physiol 315: R751–R758, 2018. doi: 10.1152/ajpregu.00076.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Wang XP, Xing CY, Zhang JX, Zhou JH, Li YC, Yang HY, Zhang PF, Zhang W, Huang Y, Long JG, Gao F, Zhang X, Li J. Time-restricted feeding alleviates cardiac dysfunction induced by simulated microgravity via restoring cardiac FGF21 signaling. FASEB J 34: 15180–15196, 2020. doi: 10.1096/fj.202001246RR. [DOI] [PubMed] [Google Scholar]
- 311. Schafer MJ, Mazula DL, Brown AK, White TA, Atkinson E, Pearsall VM, Aversa Z, Verzosa GC, Smith LA, Matveyenko A, Miller JD, LeBrasseur NK. Late-life time-restricted feeding and exercise differentially alter healthspan in obesity. Aging Cell 18: e12966, 2019. doi: 10.1111/acel.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Schroder EA, Burgess DE, Johnson SR, Ono M, Seward T, Elayi CS, Esser KA, Delisle BP. Timing of food intake in mice unmasks a role for the cardiomyocyte circadian clock mechanism in limiting QT-interval prolongation. Chronobiol Int 39: 525–534, 2022. doi: 10.1080/07420528.2021.2011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Hung HC, Yang YC, Ou HY, Wu JS, Lu FH, Chang CJ. The association between self-reported sleep quality and overweight in a Chinese population. Obesity (Silver Spring) 21: 486–492, 2013. doi: 10.1002/oby.20259. [DOI] [PubMed] [Google Scholar]
- 314. Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Arch 463: 139–160, 2012. doi: 10.1007/s00424-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435–1439, 1999. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 316. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 16: 643–653, 2008. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Broussard JL, Kilkus JM, Delebecque F, Abraham V, Day A, Whitmore HR, Tasali E. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity (Silver Spring) 24: 132–138, 2016. doi: 10.1002/oby.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 59: 2126–2133, 2010. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med 157: 549–557, 2012. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Broussard JL, Chapotot F, Abraham V, Day A, Delebecque F, Whitmore HR, Tasali E. Sleep restriction increases free fatty acids in healthy men. Diabetologia 58: 791–798, 2015. doi: 10.1007/s00125-015-3500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, Schmid SM, Lehnert H, Oster H. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS One 7: e37150, 2012. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Hou T, Wang C, Joshi S, O’Hara BF, Gong MC, Guo Z. Active time-restricted feeding improved sleep-wake cycle in db/db mice. Front Neurosci 13: 969, 2019. doi: 10.3389/fnins.2019.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM. Restricted feeding-induced sleep, activity, and body temperature changes in normal and preproghrelin-deficient mice. Am J Physiol Regul Integr Comp Physiol 298: R467–R477, 2010. doi: 10.1152/ajpregu.00557.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Wang HB, Loh DH, Whittaker DS, Cutler T, Howland D, Colwell CS. Time-restricted feeding improves circadian dysfunction as well as motor symptoms in the Q175 mouse model of Huntington’s disease. eNeuro 5: ENEURO.0431-17.2017, 2018. doi: 10.1523/ENEURO.0431-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347: 1265–1269, 2015. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Pavlou V, Lin S, Wiseman E, Varady KA. The effect of 4-h versus 6-h time restricted feeding on sleep quality, duration, insomnia severity and obstructive sleep apnea in adults with obesity. Nutr Health 28: 5–11, 2022. doi: 10.1177/02601060211002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Villanueva JE, Livelo C, Trujillo AS, Chandran S, Woodworth B, Andrade L, Le HD, Manor U, Panda S, Melkani GC. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat Commun 10: 2700, 2019. doi: 10.1038/s41467-019-10563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Zhang X, Zou Q, Zhao B, Zhang J, Zhao W, Li Y, Liu R, Liu X, Liu Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol 32: 101535, 2020. doi: 10.1016/j.redox.2020.101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Ren J, Hu D, Mao Y, Yang H, Liao W, Xu W, Ge P, Zhang H, Sang X, Lu X, Zhong S. Alteration in gut microbiota caused by time-restricted feeding alleviate hepatic ischaemia reperfusion injury in mice. J Cell Mol Med 23: 1714–1722, 2019. doi: 10.1111/jcmm.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Frazier K, Chang EB. Intersection of the gut microbiome and circadian rhythms in metabolism. Trends Endocrinol Metab 31: 25–36, 2020. doi: 10.1016/j.tem.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Choi H, Rao MC, Chang EB. Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism. Nat Rev Gastroenterol Hepatol 18: 679–689, 2021. doi: 10.1038/s41575-021-00452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Takayasu L, Suda W, Takanashi K, Iioka E, Kurokawa R, Shindo C, Hattori Y, Yamashita N, Nishijima S, Oshima K, Hattori M. Circadian oscillations of microbial and functional composition in the human salivary microbiome. DNA Res 24: 261–270, 2017. doi: 10.1093/dnares/dsx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Collado MC, Engen PA, Bandín C, Cabrera-Rubio R, Voigt RM, Green SJ, Naqib A, Keshavarzian A, Scheer F, Garaulet M. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB J 32: 2060–2072, 2018. doi: 10.1096/fj.201700697RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Kaczmarek JL, Musaad SM, Holscher HD. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr 106: 1220–1231, 2017. doi: 10.3945/ajcn.117.156380. [DOI] [PubMed] [Google Scholar]
- 335. Skarke C, Lahens NF, Rhoades SD, Campbell A, Bittinger K, Bailey A, Hoffmann C, Olson RS, Chen L, Yang G, Price TS, Moore JH, Bushman FD, Greene CS, Grant GR, Weljie AM, FitzGerald GA. A pilot characterization of the human chronobiome. Sci Rep 7: 17141, 2017. doi: 10.1038/s41598-017-17362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17: 681–689, 2015. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Weger BD, Gobet C, Yeung J, Martin E, Jimenez S, Betrisey B, Foata F, Berger B, Balvay A, Foussier A, Charpagne A, Boizet-Bonhoure B, Chou CJ, Naef F, Gachon F. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab 29: 362–382.e8, 2019. doi: 10.1016/j.cmet.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 153: 812–827, 2013. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 339. Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, Panda S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun 9: 2872, 2018. doi: 10.1038/s41467-018-05336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Bishehsari F, Engen PA, Voigt RM, Swanson G, Shaikh M, Wilber S, Naqib A, Green SJ, Shetuni B, Forsyth CB, Saadalla A, Osman A, Hamaker BR, Keshavarzian A, Khazaie K. Abnormal eating patterns cause circadian disruption and promote alcohol-associated colon carcinogenesis. Cell Mol Gastroenterol Hepatol 9: 219–237, 2020. doi: 10.1016/j.jcmgh.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, Bailey J, Myers RP, Rioux KP. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 11: 868–875.e3, 2013. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 342. Vital M, Rud T, Rath S, Pieper DH, Schlüter D. Diversity of bacteria exhibiting bile acid-inducible 7alpha-dehydroxylation genes in the human gut. Comput Struct Biotechnol J 17: 1016–1019, 2019. doi: 10.1016/j.csbj.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4: 2384, 2013. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Russell BJ, Brown SD, Siguenza N, Mai I, Saran AR, Lingaraju A, Maissy ES, Dantas Machado AC, Pinto AFM, Sanchez C, Rossitto LA, Miyamoto Y, Richter RA, Ho SB, Eckmann L, Hasty J, Gonzalez DJ, Saghatelian A, Knight R, Zarrinpar A. Intestinal transgene delivery with native E. coli chassis allows persistent physiological changes. Cell 185: 3263–3277.e15, 2022. doi: 10.1016/j.cell.2022.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernandez-Real JM, Backhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23: 850–858, 2017. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 346. Reitmeier S, Kiessling S, Clavel T, List M, Almeida EL, Ghosh TS, Neuhaus K, Grallert H, Linseisen J, Skurk T, Brandl B, Breuninger TA, Troll M, Rathmann W, Linkohr B, Hauner H, Laudes M, Franke A, Le Roy CI, Bell JT, Spector T, Baumbach J, O’Toole PW, Peters A, Haller D. Arrhythmic gut microbiome signatures predict risk of type 2 diabetes. Cell Host Microbe 28: 258–272.e6, 2020. doi: 10.1016/j.chom.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 347. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541–546, 2013. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 348. Chou CJ, Membrez M, Blancher F. Gut decontamination with norfloxacin and ampicillin enhances insulin sensitivity in mice. Nestle Nutr Workshop Ser Pediatr Program 62: 127–137, 2008. doi: 10.1159/000146256. [DOI] [PubMed] [Google Scholar]
- 349. Hwang I, Park YJ, Kim YR, Kim YN, Ka S, Lee HY, Seong JK, Seok YJ, Kim JB. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J 29: 2397–2411, 2015. doi: 10.1096/fj.14-265983. [DOI] [PubMed] [Google Scholar]
- 350. Suárez-Zamorano N, Fabbiano S, Chevalier C, Stojanović O, Colin DJ, Stevanović A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, Ilievska M, Montet X, Seimbille Y, Hapfelmeier S, Trajkovski M. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med 21: 1497–1501, 2015. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Wichmann A, Allahyar A, Greiner TU, Plovier H, Lundèn GO, Larsson T, Drucker DJ, Delzenne NM, Cani PD, Bäckhed F. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 14: 582–590, 2013. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 352. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6: 414–421, 2007. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 354. Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, Dilag-Penilla SA, Dyar KA, Baldi P, Sassone-Corsi P. Reprogramming of the circadian clock by nutritional challenge. Cell 155: 1464–1478, 2013. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. de Goede P, Wüst RC, Schomakers BV, Denis S, Vaz FM, Pras-Raves ML, van Weeghel M, Yi CX, Kalsbeek A, Houtkooper RH. Time-restricted feeding during the inactive phase abolishes the daily rhythm in mitochondrial respiration in rat skeletal muscle. FASEB J 36: e22133, 2022. doi: 10.1096/fj.202100707R. [DOI] [PubMed] [Google Scholar]
- 356. Chung KW, Chung HY. The effects of calorie restriction on autophagy: role on aging intervention. Nutrients 11: 2923, 2019. doi: 10.3390/nu11122923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Ulgherait M, Midoun AM, Park SJ, Gatto JA, Tener SJ, Siewert J, Klickstein N, Canman JC, Ja WW, Shirasu-Hiza M. Circadian autophagy drives iTRF-mediated longevity. Nature 598: 353–358, 2021. doi: 10.1038/s41586-021-03934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Mizushima N, Murphy LO. Autophagy assays for biological discovery and therapeutic development. Trends Biochem Sci 45: 1080–1093, 2020. doi: 10.1016/j.tibs.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 359. Bensalem J, Hattersley KJ, Hein LK, Teong XT, Carosi JM, Hassiotis S, Grose RH, Fourrier C, Heilbronn LK, Sargeant TJ. Measurement of autophagic flux in humans: an optimized method for blood samples. Autophagy 17: 3238–3255, 2021. doi: 10.1080/15548627.2020.1846302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, Thompson D. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr 100: 539–547, 2014. doi: 10.3945/ajcn.114.083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Yoshimura E, Hatamoto Y, Yonekura S, Tanaka H. Skipping breakfast reduces energy intake and physical activity in healthy women who are habitual breakfast eaters: randomized crossover trial. Physiol Behav 174: 89–94, 2017. doi: 10.1016/j.physbeh.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 362. Clayton DJ, Barutcu A, Machin C, Stensel DJ, James LJ. Effect of breakfast omission on energy intake and evening exercise performance. Med Sci Sports Exerc 47: 2645–2652, 2015. doi: 10.1249/MSS.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 363. Kral TV, Whiteford LM, Heo M, Faith MS. Effects of eating breakfast compared with skipping breakfast on ratings of appetite and intake at subsequent meals in 8- to 10-y-old children. Am J Clin Nutr 93: 284–291, 2011. doi: 10.3945/ajcn.110.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Levitsky DA, Pacanowski CR. Effect of skipping breakfast on subsequent energy intake. Physiol Behav 119: 9–16, 2013. doi: 10.1016/j.physbeh.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 365. Chowdhury EA, Richardson JD, Tsintzas K, Thompson D, Betts JA. Carbohydrate-rich breakfast attenuates glycaemic, insulinaemic and ghrelin response to ad libitum lunch relative to morning fasting in lean adults. Br J Nutr 114: 98–107, 2015. doi: 10.1017/S0007114515001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Bonnet JP, Cardel MI, Cellini J, Hu FB, Guasch-Ferré M. Breakfast skipping, body composition, and cardiometabolic risk: a systematic review and meta-analysis of randomized trials. Obesity (Silver Spring) 28: 1098–1109, 2020. doi: 10.1002/oby.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 81: 388–396, 2005. doi: 10.1093/ajcn.81.2.388. [DOI] [PubMed] [Google Scholar]
- 368. Nas A, Mirza N, Hägele F, Kahlhöfer J, Keller J, Rising R, Kufer TA, Bosy-Westphal A. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr 105: 1351–1361, 2017. doi: 10.3945/ajcn.116.151332. [DOI] [PubMed] [Google Scholar]
- 369. Verboeket-van de Venne WP, Westerterp KR. Influence of the feeding frequency on nutrient utilization in man: consequences for energy metabolism. Eur J Clin Nutr 45: 161–169, 1991. [PubMed] [Google Scholar]
- 370. Taylor MA, Garrow JS. Compared with nibbling, neither gorging nor a morning fast affect short-term energy balance in obese patients in a chamber calorimeter. Int J Obes Relat Metab Disord 25: 519–528, 2001. doi: 10.1038/sj.ijo.0801572. [DOI] [PubMed] [Google Scholar]
- 371. Kobayashi F, Ogata H, Omi N, Nagasaka S, Yamaguchi S, Hibi M, Tokuyama K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes Res Clin Pract 8: e201–e298, 2014. doi: 10.1016/j.orcp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 372. Ogata H, Kayaba M, Tanaka Y, Yajima K, Iwayama K, Ando A, Park I, Kiyono K, Omi N, Satoh M, Tokuyama K. Effect of skipping breakfast for 6 days on energy metabolism and diurnal rhythm of blood glucose in young healthy Japanese males. Am J Clin Nutr 110: 41–52, 2019. doi: 10.1093/ajcn/nqy346. [DOI] [PubMed] [Google Scholar]
- 373. Richter J, Herzog N, Janka S, Baumann T, Kistenmacher A, Oltmanns KM. Twice as high diet-induced thermogenesis after breakfast vs dinner on high-calorie as well as low-calorie meals. J Clin Endocrinol Metab 105: dgz311, 2020. doi: 10.1210/clinem/dgz311. [DOI] [PubMed] [Google Scholar]
- 374. Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr 66: 239–246, 1997. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 375. Kahleova H, Belinova L, Malinska H, Oliyarnyk O, Trnovska J, Skop V, Kazdova L, Dezortova M, Hajek M, Tura A, Hill M, Pelikanova T. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia 57: 1552–1560, 2014. doi: 10.1007/s00125-014-3253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 21: 421–423, 2013. doi: 10.1002/oby.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Fong M, Caterson ID, Madigan CD. Are large dinners associated with excess weight, and does eating a smaller dinner achieve greater weight loss? A systematic review and meta-analysis. Br J Nutr 118: 616–628, 2017. doi: 10.1017/S0007114517002550. [DOI] [PubMed] [Google Scholar]
- 378. Keim NL, Van Loan MD, Horn WF, Barbieri TF, Mayclin PL. Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J Nutr 127: 75–82, 1997. doi: 10.1093/jn/127.1.75. [DOI] [PubMed] [Google Scholar]
- 379. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 21: 2504–2512, 2013. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 380. Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Beneficial effect of high energy intake at lunch rather than dinner on weight loss in healthy obese women in a weight-loss program: a randomized clinical trial. Am J Clin Nutr 104: 982–989, 2016. doi: 10.3945/ajcn.116.134163. [DOI] [PubMed] [Google Scholar]
- 381. Lombardo M, Bellia A, Padua E, Annino G, Guglielmi V, D’Adamo M, Iellamo F, Sbraccia P. Morning meal more efficient for fat loss in a 3-month lifestyle intervention. J Am Coll Nutr 33: 198–205, 2014. doi: 10.1080/07315724.2013.863169. [DOI] [PubMed] [Google Scholar]
- 382. Rabinovitz HR, Boaz M, Ganz T, Jakubowicz D, Matas Z, Madar Z, Wainstein J. Big breakfast rich in protein and fat improves glycemic control in type 2 diabetics. Obesity (Silver Spring) 22: E46–E54, 2014. doi: 10.1002/oby.20654. [DOI] [PubMed] [Google Scholar]
- 383. Jakubowicz D, Barnea M, Wainstein J, Froy O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin Sci (Lond) 125: 423–432, 2013. doi: 10.1042/CS20130071. [DOI] [PubMed] [Google Scholar]
- 384. Versteeg RI, Ackermans MT, Nederveen AJ, Fliers E, Serlie MJ, la Fleur SE. Meal timing effects on insulin sensitivity and intrahepatic triglycerides during weight loss. Int J Obes 42: 156–162, 2018. doi: 10.1038/ijo.2017.199. [DOI] [PubMed] [Google Scholar]
- 385. Meessen EC, Andresen H, van Barneveld T, van Riel A, Johansen EI, Kolnes AJ, Kemper EM, Olde Damink SW, Schaap FG, Romijn JA, Jensen J, Soeters MR. Differential effects of one meal per day in the evening on metabolic health and physical performance in lean individuals. Front Physiol 12: 771944, 2021. doi: 10.3389/fphys.2021.771944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Jensen TL, Kiersgaard MK, Sørensen DB, Mikkelsen LF. Fasting of mice: a review. Lab Anim 47: 225–240, 2013. doi: 10.1177/0023677213501659. [DOI] [PubMed] [Google Scholar]
- 387. Hamprecht B, Nüssler C, Lynen F. Rhythmic changes of hydroxymethylglutaryl coenzyme A reductase activity in livers of fed and fasted rats. FEBS Lett 4: 117–121, 1969. doi: 10.1016/0014-5793(69)80210-3. [DOI] [PubMed] [Google Scholar]