Abstract
Background
Insulin resistance (IR) is the main risk factor for developing type 2 diabetes. Both strength training (ST) and photobiomodulation therapy (PBMt) reduce IR, but the effect of combining different volumes of ST with PBMt is unknown.
Methods
Overweight/obese individuals will be assigned to 4 groups (n = 12/group): ST with volume following international guidelines (3 sets per exercise – high volume) or one-third of this volume (1 set per exercise – low volume), combined with PBMt or placebo. ST will be performed for 20 sessions over 10 weeks and will consist of 7 exercises. The PBMt will be applied after training sessions using blankets with light emitters (LEDs) placed over the skin on the frontal and the posterior region of the body, following the parameters recommended by the literature. The placebo group will undergo an identical procedure, but blankets will emit insignificant light. To measure plasma glucose and insulin concentrations, oral glucose tolerance tests (OGTT) will be performed before and after the training period. Thereafter, IR, the area under the curve of glucose and insulin, and OGTT-derived indices of insulin sensitivity/resistance will be calculated.
Expected impact on the field
This study will determine the effects of different ST volumes on IR and whether the addition of PBMt potentiates the effects of ST. Because previously sedentary, obese, insulin-resistant individuals might not comply with recommended volumes of exercise, the possibility that adding PBMt to low-volume ST enhances ST effects on IR bears practical significance.
Keywords: Resistance training, Type 2 diabetes, Light therapy, Insulin sensitivity
Highlights
-
•
Strength training (ST) and photobiomodulation therapy (PBMt) improve insulin resistance (IR).
-
•
Combining ST and PBMt has not been investigated for improvement of IR.
-
•
This study will determine the effects of different volumes of ST and PBMt on IR.
-
•
The results might aid in prescribing ST and PBMt for IR individuals.
1. Introduction
Diabetes mellitus (DM) is a set of diseases characterized by chronic hyperglycemia [1]. It was estimated that in 2017 there were 451 million cases of DM worldwide, which is expected to rise to 693 million in 2045 [2]. Also in 2017, DM was responsible for 9.9% of deaths from all causes and expenditure on health care was 850 billion American dollars (USD) [2]. In Brazil, 7.4% Brazilians over 18 years of age were diabetic in 2019 [3] and the costs of hospitalizations, outpatient procedures, and medications attributable to DM in the national health care system in 2018 were more than 1 billion Brazilian reais [4]. Type 2 DM (T2DM) is the most prevalent form of DM and accounts for 90–95% of cases [1,5].
The etiology of T2DM is related to insulin resistance (IR) [6]. In IR, the effects insulin are attenuated, which compared to the normal condition, increases hepatic glucose production, and reduces glucose uptake by adipose tissue and skeletal muscle [[7], [8], [9], [10]]. As a compensatory response, pancreatic beta cells produce more insulin to control plasma glucose concentration. However, if IR persists, over time, insulin production may become insufficient to maintain normal blood glucose or the pancreas may lose its ability to produce insulin. In either case, hyperglycemia will prevail, leading to T2DM [11]. Therefore, the prevention and treatment of IR are essential to prevent and control the progression of T2DM and its consequences.
Several studies have demonstrated that strength training effectively reduces IR [[12], [13], [14], [15]]. Specifically, improvement in glucose metabolism has been observed in response to strength training in T2DM patients [12,16,17], in pre-diabetic [18], obese [19,20], overweight [21] and healthy individuals [13]. In addition, strength training appears to have beneficial effects on IR by increasing fat-free mass [22], reducing visceral adiposity [23], reducing inflammation [24,25] and leading to mitochondrial adaptations [[26], [27], [28], [29]]. Indeed, because of their beneficial effects on improving IR and other health parameters, international guidelines recommend that the general population [[30], [31], [32], [33]] and T2DM patients [[34], [35], [36]] engage in strength training.
In addition to strength training, other non-pharmacological and non-invasive strategies improve IR. In this context, light therapy, known as phototherapy or photobiomodulation therapy (PBMt) [37] is an interesting alternative. PBMt utilizes non-ionizing forms of light sources, including lasers, light emitting diodes (LEDs), and broadband light, in the visible and infrared spectrum to induce biological effects [38,39]. Although the mechanisms by which PBMt acts are not entirely known, studies suggest it elicits positive effects by improving mitochondrial function [39]. Furthermore, improvements in inflammatory parameters are also reported with PBMt [40]. Recently, different research groups have demonstrated that treatment with PBMt reduced IR and improved glucose metabolism in mice fed a high-fat diet [[41], [42], [43], [44], [45]] and in T2DM patients [46]. Furthermore, results showed there was an improvement in mitochondrial function in skeletal muscle, greater activation of the insulin pathway in adipose tissue and skeletal muscle, less inflammation in adipose tissue, and less visceral adiposity [41,42]. Therefore, PBMt was suggested to be a potentially effective tool for improving IR [47].
Interestingly, studies have shown that PBMt can enhance the effects of exercise on IR. For example, da Silveira Campos et al. [48] showed that 4 months of exercise training combined with PBMt reduced circulating insulin concentration in obese women, which was not observed with exercise training alone. Furthermore, the same group of researchers [49,50] used a similar protocol and sample and showed the delta reduction in IR was greater in the exercise training + PBMt group than in the group that only exercised. These results indicate that PBMt can potentiate the effects of exercise training on IR. Importantly, in the studies mentioned above [[48], [49], [50]], the exercise sessions were a combination of 30 min of aerobic exercise and 30 min of strength exercise. Thus, no studies in the literature have investigated the effect of strength training alone with PBMt on IR, even though PBMt has been shown to potentiate the effects of strength training on other adaptation parameters (e.g. strength and hypertrophy) [[51], [52], [53], [54]]. Thus, it is plausible to hypothesize that PBMt might also potentiate the effects of strength training on IR, although this has not yet been investigated previously.
Although the combination of strength training with PBMt is potentially beneficial for improving IR, some factors should be considered. For previously sedentary or physically inactive insulin-resistant, obese individuals, performing aerobic exercise can be difficult, uncomfortable, even painful [55], and for these subjects, strength training might be a viable option [12]. Conversely, even if the individual is able to perform strength exercise, the recommended volume of strength exercise (6–10 exercises, 3 sets/exercise) by international guidelines [34] may be intangible for these individuals, which can result in demotivation and low adherence to training [56]. It is important to emphasize that the literature is still not unanimous in pointing out the best prescription for strength exercise to treat IR [15], and in particular there is ongoing discussion about the appropriate volume (number of exercises x sets/exercise x repetitions/set) of training to be prescribed [57,58]. Last but not least, lack of time is one of the reasons most commonly cited by people for not exercising regularly [59,60]. Keeping all the above factors in mind, it would be interesting to know the effects of strength training, consisting of exercise sessions with less volume than recommended (e.g. 66.6% less), and by consequence, less time commitment, combined or not with PBMt, on the reduction of IR compared to traditional strength training volume. This question has major practical implications for the rehabilitation and health professions regarding the prescription of strength training for individuals who cannot reach the recommended training volume to improve IR.
1.1. Objective
The primary objective of this study is to investigate the effect of different strength training volumes combined with PBMt on IR. Both strength training and PBMt improve IR when performed alone, and the combination of PBMt with strength training enhances the traditional adaptations of the latter. Thus, the study hypothesis is that the association of strength training with PBMt will improve IR more than strength training alone. Furthermore, by reducing the strength training volume to one-third of the original, the improvement in IR will be attenuated. Conversely, the association of reduced training volume with PBMt will induce greater improvement in IR than training with reduced volume without PBMt.
1.2. Secondary objectives
The secondary objectives will be to investigate the effects of strength training prescribed with different volumes combined with PBMt on visceral adiposity, fat mass and fat-free mass and strength. The hypothesis is that strength training will lead to positive adaptations in the previous variables, and reducing training volume will attenuate these adaptations. Moreover, PBMt combined with strength training will induce greater adaptation than strength training alone.
2. Methods
Ethics approval
This study received approval from the ethics committee of the local university (certificate number CAAE 45109621.7.0000.5108). This study complies with the Declaration of Helsinki. The present study was prospectively registered in a clinical trial registry (ReBEC #11453 - https://ensaiosclinicos.gov.br/rg/RBR-7rtcpp6). Any modifications to the protocol will be submitted to the ethics committee, followed by an updating of the trial registry.
2.1. Description of participants
The presence of overweight and obesity are determinants for IR [61,62]. Therefore, inclusion criteria are individuals of both sexes with overweight (body mass index - BMI>25 kg/m2) or obesity (BMI>30 kg/m2), aged between 18 and 60 years, stable body mass over the past 3 months, insufficiently physically active (<150 min/week of moderate to intense physical activity) [63], able to perform physical activities [[64], [65], [66]]. Exclusion criteria are individuals with diabetes or another metabolic disease, those who report using drugs with an effect on metabolism, anti-inflammatory medications, or anabolic steroids. Pregnant women will be excluded from the study, but contraceptive use will not be an exclusion criterion. The project will be publicized by fixing posters on appropriate murals in the university buildings, by distributing folders at health units in the city, through posters and advertisements distributed at commercial points in the city, and by word of mouth. After volunteering, individuals will be informed about the objectives and methodological procedures of the study, as well as the possible risks, discomforts and benefits related to participation in the research. Participation in the study as a volunteer will be subject to signing the “Informed Consent Form".
2.2. Study design
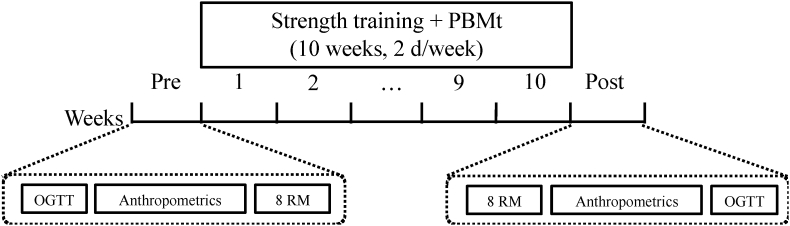
This study will be an assessor-, therapists-, and participant-tripled-blinded, randomized, placebo-controlled trial. First, participants will undergo a baseline oral glucose tolerance test (OGTT) and will be submitted to anthropometric analyses to measure body mass, height, waist circumference, fat mass, fat-free mass and visceral fat mass, and strength assessments. Then, overweight/obese individuals will be randomly assigned (using the website https://www.randomizer.org) into four groups: 20 strength training sessions over 10 weeks with volume according to international guidelines (3 sets per exercise – high volume) or one-third of this volume (1 set per exercise – low volume), undergoing post-exercise session treatment with PBMt or placebo: high-Pla (n = 12); low-Pla (n = 12); high-PBMt (n = 12) and low-PBMt (n = 12). After the intervention period, post-training assessments will be performed. Fig. 1 illustrates the timeline of the experimental protocol.
Fig. 1.
Illustration of the experimental protocol timeline. PBMt: photobiomodulation therapy; OGTT: oral glucose tolerance test; 8 RM: strength tests.
2.3. Outcome measures
2.3.1. Primary outcomes
Insulin resistance - Oral Glucose Tolerance Test. After at least 48 h of physical inactivity, and after 8–10 h of overnight fast subjects will report to the laboratory. A catheter will be inserted into the antecubital vein and a blood sample will be taken (minute 0). Subsequently, they will ingest 75 g of glucose in 300 mL of water and blood samples will be taken every 30 min until 120 min after ingestion of the glucose solution, totaling 5 withdrawals (0, 30, 60, 90 and 120 min) of 5 ml each. Plasma glucose and insulin concentrations will be measured in all samples. With the results of glucose and insulin at time 0 min, IR will be calculated from the homeostasis model of IR (HOMA-IR) using the formula glucose (mmol) x insulin (μU/mL) ÷ 22.5 [67] and insulin sensitivity will be calculated using the quantitative insulin sensitivity check index (QUICKI) [68]. Other indexes of insulin sensitivity and IR will be calculated from the results of the OGTT [69], such as the Oral Glucose Insulin Sensitivity index [70], the Matsuda index [71], Cederholm's insulin sensitivity index [72], skeletal muscle insulin sensitivity index [73,74], the glucose-stimulated insulin secretion index [75], oral disposition index [76,77], the Gutt index [78], the Avignon et al. index [79], Belfiore et al. index [80], the Stumvoll et al. index [81], and the McAuley et al. index [82]. Furthermore, total glucose and insulin area under the curve (AUC) will be calculated using the trapezoidal method [83]. Post-training OGTT will be performed 48 h after the strength test session, and 24 h after the anthropometric measurements. Individuals whose fasting glucose result (0 min) is above 125 mg/dL and/or the 120-min glucose result is above 199 mg/dL will be excluded from the study.
2.3.2. Secondary outcomes
Anthropometric measurements. At least 24 h after the pre-training OGTT, and after a standardized breakfast, individuals will have body mass and height measured using an analog scale (Welmy, model 110, 0.1 kg precision) with a stadiometer attached (0.5 cm precision) to calculate the BMI and the measurement of the volunteers' waist circumference will be taken. Then, body composition will be analyzed using Dual-energy X-ray Absorptiometry (DEXA, Lunar, iDXA Advanced). Individuals will be positioned on the equipment and the manufacturer's instructions will be followed for analysis of fat mass, fat-free mass and visceral fat mass. Anthropometric measurements will be retaken after the strength training period, 24 h before OGTT and 24 h after the strength test session.
Strength tests. After two familiarization sessions [[84], [85], [86]], subjects will perform the strength test. Subjects will perform 8 RM [87,88] to assess the effect of training on strength, and the tests will be repeated 48 h after the last training session.
Assessment of total food intake and composition. Volunteers will be instructed not to change their eating habits during the study period. To verify the caloric intake (calories) and composition (macronutrients) of foods, a diet log will be used to monitor two weekdays and one weekend day, as suggested by Willet and Stampfer [89], and previously carried out by our research group [74]. The diet record will be done in the 1st and in the 10th weeks of training. The data obtained will be converted into weight (kg) or volume (ml) and analyzed using commercial food analysis software (DietPro R, version 5.7, AS Systems, 2013).
2.4. Assignment of interventions
2.4.1. Random allocation
The participants will be randomly allocated to one of four groups (1) high-Pla (overweight/obese group that will perform strength training with 3 sets per exercise and will undergo placebo treatment); (2) low-Pla (overweight/obese group that will perform strength training with 1 set per exercise and will undergo placebo treatment); (3) high-PBMt (overweight/obese group that will perform strength training with 3 sets per exercise and will undergo treatment with PBMt); (4) low-PBMt (overweight/obese group that will perform strength training with 1 set per exercise and will undergo treatment with PBMt). The randomization will be generated by the website https://www.randomizer.org and performed by a researcher not involved in the participants' recruitment, treatment, or assessment. This researcher will be instructed not to disclose the programmed intervention to the therapist, participants, or other researchers until the completion of the study. In addition, the participants and therapist will be blinded throughout the treatment (see Blinding section below). Concealed allocation will be achieved using sequentially numbered, sealed, opaque envelopes.
2.4.2. Blinding
This will be a triple (participants, therapist, assessor)-blinded study. To ensure proper blinding, the PBMt device will be previously coded for the active or placebo mode by a researcher not involved in the randomization, training, PBMt application, or assessment. The active and placebo modes are identical, emit the same sounds, and present the same information on the display. Moreover, as the device produces a non-significant amount of heat, and participants will wear reflective glasses during the application, they will be blinded to PBMt (active or placebo) treatment. The therapist responsible for the PBMt application will be blinded to participants’ allocation (high-volume or low-volume strength training, and PBMt or placebo), and to pre-training measure outcomes. The therapists responsible for training will be blinded to PBMt (active or placebo) treatment, and to pre-training measure outcomes. The researcher responsible for assessing outcomes will be blinded to randomization. By the end of the experimental protocol, participants, the therapist responsible for PBMt, the therapist responsible for conducting the training sessions, and the researcher responsible for assessing outcomes will be asked about each participant group to confirm blinding. Blinding effectiveness will be tested using the χ2 test.
2.5. Interventions
Strength Training. The prescription of strength training will be based on international guidelines for the general population and for diabetics, and on recent studies investigating the effect of strength training on IR in overweight/obese individuals [21,32,34,90]. The training will be conducted out for 10 weeks, on 2 non-consecutive days per week. It will consist of 7 exercises per session [91], with 1 (low volume) or 3 (high volume) sets per exercise, and will be of moderate (12–15 repetitions) to high (6–8 repetitions) intensity. Each repetition will consist of 1 s in the concentric phase and 2 s in the eccentric phase (controlled with the aid of a metronome), with each set performed until momentary fatigue (defined as the individual's incapacity to maintain the duration of the concentric or eccentric phases in 2 consecutive repetitions). After each set, subjects will be instructed to rate their effort using the OMNI-RPE scale [92]. If needed, weight will be adjusted so prescribed repetition range is maintained. The interval between sets will be from 90 to 120 s. In all sets, the number of repetitions actually performed and the weight lifted in each set will be recorded so that the total training volume can be calculated (sets x repetitions x weight) [93]. If an individual misses a session, they will allowed to reschedule. Any individual who misses (without rescheduling) 3 non-consecutive sessions, or 2 consecutive sessions will be excluded from the study. Table 1 shows the training prescription.
Table 1.
Strength training prescription.
| Duration | 10 weeks |
|---|---|
| Frequency | 2 non-consecutive days per week |
| Number of Exercises | 7 exercises |
| Intensity | Moderate-high (to concentric failure) |
| Volume (number of sets) | 1 or 3 sets per exercise |
| Repetitions per set | Weeks 1–2: 12-15 |
| Weeks 3–5: 10-12 | |
| Weeks 6–7: 8-10 | |
| Weeks 8–10: 6-8 | |
| Repetition tempo (con:ecc) | 1:2 s |
| Rest intervals between sets | 90–120 s |
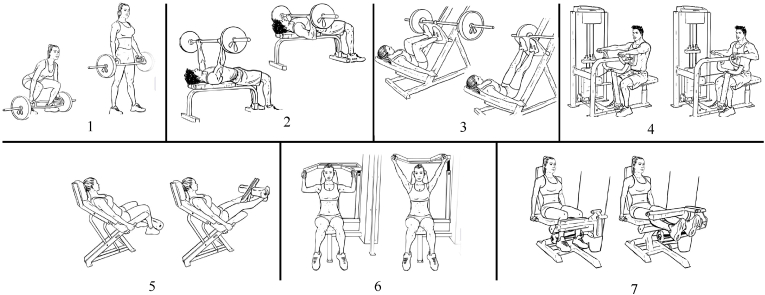
The 7 exercises will be: 1) hex bar deadlift, 2) bench press, 3) leg press, 4) seated row, 5) leg extension, 4) shoulder press, and 7) leg curl. Fig. 2 illustrates these exercises.
The training will be carried out in a weightlifting laboratory and will always be accompanied by a certified professional who will be blinded to participant allocation (PBMt or placebo; see Blinding section above). The training will be carried out at a specific time, according to the availability of individuals, without the presence of other people not involved in the project. Subjects will be asked to refrain from further strength training during the study period and to maintain their usual physical activity and diet habits. In the first and last week of training, a diet log will verify caloric intake and diet composition (see Assessment of total food intake and composition above).
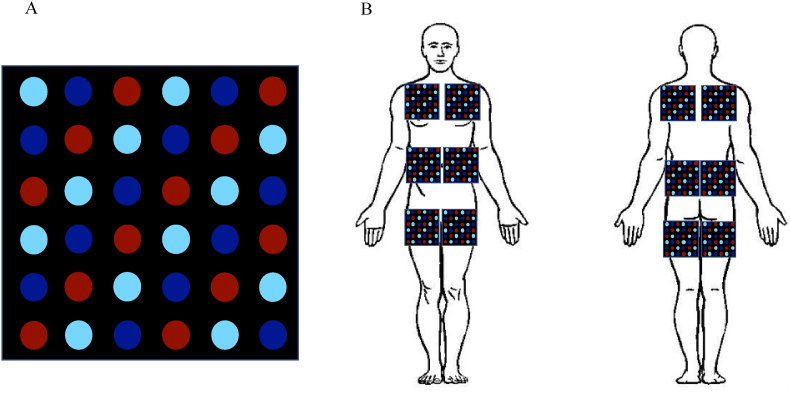
Photobiomodulation therapy (PBMt). The PBMt parameters will be based on randomized clinical trials [[48], [49], [50],[94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110]]. The application of PBMt will start immediately after all training sessions using custom-made flexible blankets in black color measuring 18 × 18 cm, with 6 columns of 6 LED diodes each (total of 36 diodes). Red (12 × 660 nm) and infrared (12x850 and 12 × 940 nm) wavelength diodes will be used, with the diodes placed in an intercalated fashion (Fig. 3A). The diodes’ power output will be measured monthly during the experimental period using an optical energy meter PM100USB Thorlabs® fitted with a sensor S121C. During the application of PBMt, individuals will wear shorts (men) and shorts and tops (women) for the correct positioning of the blankets. Participants will lie down on a stretcher and the positioning of the blankets will be over the pectoralis major muscle, the abdominal region and the quadriceps (anterior view) and over the upper back, lower back and the posterior region of the thigh (posterior view) (Fig. 3B).
Fig. 2.
Exercises that will be prescribed during strength training. 1) hex bar deadlift, 2) bench press, 3) leg press, 4) seated row, 5) leg extension, 6) shoulder press, and 7) leg curl.
Fig. 3.
A) Distribution of diodes in the blanket. B) Schematic illustration of the positioning of the blankets on the skin. Red = 660 nm diode; dark blue = 850 nm diode; light blue = 940 nm diode. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Each diode will emit optical power equivalent to 70 mW, and the blanket will be in contact with the skin for 193 s, therefore, 13.51 J will be transferred per diode, totaling 486.36 J (36 diodes x 13.51 J per diode) per application site. The total energy transferred per session will be 5836.32 J (12 sites x 486.36 J per site). The PBMt parameters are shown in Table 2. In the groups receiving placebo treatment, the blanket will be positioned identically, but the optical power will be < 1 mW per diode, such that the energy transfer will be < 0.1 J per diode and <4 J per application site or <50 J per session (<1% of energy transferred in PBMt). In addition, all individuals will wear reflective glasses during the application of PBMt so that they will not be able to perceive the light on. All PBMt sessions will be in a private room, without people other than the team member administering the therapy and the individual.
Table 2.
Photobiomodulation therapy parameters.
| Type of light emitting | LEDs |
|---|---|
| Wavelengths (nm) | 660, 850, and 940 |
| Number of diodes per blanket | 12 of each wavelength – 36 total |
| Total area of the blanket (cm2) | 324 |
| Number of sites stimulated | 12 (6 in the front and 6 in the back) |
| Optical power per diode (mW) | 70 |
| Application time (sec) | 193 |
| Energy Transferred per Diode (joules) | 13.51 |
| Energy transferred per blanket (joules) | 486.36 |
| Energy density per blanket (J/cm2) | 1.50 |
| Energy transferred per session (joules) | 5836.32 |
| Application method | Contact |
2.6. Statistics
2.6.1. Sample size
The sample size was calculated using data available from Ismail et al. [21] for the result of the Cederholm insulin sensitivity index for that study, with the G*Power program (Heinrich-Heine-Universität Düsseldorf, Germany, version 3.1.9.6), entering the parameters one-tailed analysis, effect size of 0.6, alpha error probability of 0.05 and power (probability of error type 1-beta) of 0.80. With these parameters, 11 individuals per group will be needed. Due to expected dropout around 30%, we will recruit initially a total of 70 individuals, in order to have 48 (or 12 per group) individuals on the post-test analysis.
2.6.2. Data analysis
The statistical analysis will be conducted following the principles of intention-to-treat analysis [111]. Baseline data will be used to assess the comparability of the treatment groups. These variables also will be compared between participants who withdraw from the study and those who remain. Data will be expressed as mean and standard error (SE) with 95% confidence intervals (CI). For data normality analysis we will perform the Shapiro-Wilk test. For normally distributed data, results will be analyzed using analysis of variance with repeated measures with two sources of variation (training x PBMt). In case of significant main effect, posthoc Tukey will be used. When necessary, the Kruskal-Wallis or Friedman's test, followed by Dunn's post hoc test will be used for nonparametric data. The effect size will be calculated and interpreted as follows: 0.2 = small effect, 0.5 = medium effect and ≥0.8 = large effect [112,113]. The significance level adopted will be 5%. Analyzes will be performed using the Prism program (GraphPad Software, San Diego, CA-USA – version 8.4.0). The trial will be reported following the CONSORT guidelines. The statistician will be blinded to the group allocation until the completion of the analyses.
2.7. Funding and role of funding source
This study is supported by National Council for Scientific and Technological Development (CNPQ: Grant#407975/2018-7 and # 402091/2021-3) and by the Minas Gerais State Agency for Research and Development (FAPEMIG: Grant# APQ-00008-22). The funders played no role in this study's design, conduct, or reporting.
3. Discussion
3.1. Potential impact and significance of the study
The appropriate “dose” (i.e. volume) of strength exercise to improve IR has not been established [57,58]. Thus, investigating to what extent reducing training (to one-third of the recommended volume) will affect the improvement in IR is of great practical value. This is particularly important, because reducing training volume to one-third means reducing time commitment by a similar amount. So, as the most common reason reported for not regularly engaging in exercise is “lack of time” [59,60], the present study might show that reduced-volume strength training could be a time-efficient mode for improving IR.
Furthermore, it has been reported that PBMt potentiates strength training adaptations [[51], [52], [53], [54]]. Then, as strength training improves IR [[12], [13], [14], [15],29], PBMt might also prove effective in potentiating this adaptation. Suppose this study demonstrates that this association is better than strength training alone for improving IR. In that case, this can be particularly important for individuals who cannot/will not adhere to the recommended amount of exercise but would benefit from greater improvement in IR.
3.2. Strengths and weaknesses
Strengths. This study will be a triple-blinded, placebo-controlled, randomized trial. Furthermore, to ensure the high quality of this trial, we will employ principles of intention-to-treat analysis [111]. Also, the PBMt parameters that will be used have been repeatedly reported to potentiate the positive effects of strength training in humans [[51], [52], [53], [54]], and were recently reported to exert positive effects on glucose metabolism in T2DM patients [46]. Finally, the strength training protocol that will be prescribed is based on international guidelines [21,32,34,90].
Weaknesses. Assessing both men and women and including women taking oral contraceptives might increase data variability. However, we believe this design increases the ecological validity of the study. Furthermore, individuals with potentially different levels of IR might be included in this study, and greater exercise-induced improvement in IR has been observed in individuals with higher levels of baseline of IR [114,115]. Thus, this factor can also increase results variability. Finally, participants might not carry the sets to momentary fatigue, which has been suggested to be important for resistance exercise-induced improvement in glucose metabolism [116]. However, the therapist responsible for conducting the training will verbally motivate participants throughout the session to guarantee appropriate effort. Furthermore, the OMNI-RPE scale will assess subjects' perceived effort, providing information on proximity to failure.
3.3. Contribution to the health professional
Aerobic exercise is usually the first choice of exercise prescription for treating IR [117]. However, as stated previously, performing moderate to high-intensity aerobic exercise might be contraindicated for some individuals with chronic diseases, and may not be feasible for previously sedentary, insulin resistant, obese individuals [55]. Thus, the therapist and rehabilitation professional can take advantage of the results from the present study to prescribe strength training as an alternative to aerobic exercise. Also, if the patient lacks time, motivation, or capacity to adhere to a strength training prescription with traditional volume, the therapist and rehabilitation professional can use the results from the present study to prescribe a reduced-volume strength training protocol. Last, but certainly not least, the results from this study can show that associating strength training and PBMt might induce greater improvements in IR. Thus, the therapist and rehabilitation professional can make clinical use of this association in patients that would benefit from a more aggressive improvement in IR.
Authors' contributions
Flávio de Castro Magalhães, Cleber Ferraresi, Valmor A Tricoli, Fabiano Trigueiro Amorim and Zachary A Mang contributed to the concept and design of the study, and established the hypothesis. Flávio de Castro Magalhães, Cleber Ferraresi, Valmor A Tricoli, Fabiano Trigueiro Amorim and Zachary A Mang contributed significantly to the writing of the manuscript. Marco Fabrício Dias-Peixoto, Ricardo Cardoso Cassilhas, Elizabethe Adriana Esteves, Pedro Paulo Ribeiro Ferreira and Luís Filipe Rocha Silva performed critical revisions of the manuscript. All authors read and approved the final version of the manuscript.
Clinical trial registry number
ReBEC #11453 ()
Conflict of interest
None declared.
Data availability
No data was used for the research described in the article.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Brasil . Secretaria de Vigila^ncia em Saúde, Departamento de Análise em Saúde e Vigila^ncia de Doenças não Transmissíveis; Brasília: 2020. Vigitel Brasil 2019 : vigila^ncia de fatores de risco e proteção para doenças cro^nicas por inquérito telefo^nico : estimativas sobre freque^ncia e distribuição sociodemografica de fatores de risco e proteção para doenças cro^nicas nas capitais, Ministério da Saúde. [Google Scholar]
- 4.Nilson E.A.F., Andrade R. da C.S., de Brito D.A., Michele Lessa de O. vol. 44. Revista Panamericana de Salud Pública; 2018. 2020. p. 1. (Custos atribuíveis a obesidade, hipertensão e diabetes no Sistema Único de Saúde, Brasil). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 6.Who . 2006. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. [Google Scholar]
- 7.Petersen M.C., Shulman G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaribeygi H., Farrokhi F.R., Butler A.E., Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J. Cell. Physiol. 2019;234:8152–8161. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 11.Weyer C., Bogardus C., Mott D.M., Pratley R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Codella R., Ialacqua M., Terruzzi I., Luzi L. May the force be with you: why resistance training is essential for subjects with type 2 diabetes mellitus without complications. Endocrine. 2018;62:14–25. doi: 10.1007/s12020-018-1603-7. [DOI] [PubMed] [Google Scholar]
- 13.Miller W.J., Sherman W.M., Ivy J.L. Effect of strength training on glucose tolerance and post-glucose insulin response. Med. Sci. Sports Exerc. 1984;16:539–543. doi: 10.1249/00005768-198412000-00003. [DOI] [PubMed] [Google Scholar]
- 14.McLeod J.C., Stokes T., Phillips S.M. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Ye W., Chen Q., Zhang Y., Kuo C.-H., Korivi M. Resistance exercise intensity is correlated with attenuation of HbA1c and insulin in patients with type 2 diabetes: a systematic review and meta-analysis. Int. J. Environ. Res. Publ. Health. 2019;16:140. doi: 10.3390/ijerph16010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldi J.C., Snowling N. Resistance training improves glycaemic control in obese type 2 diabetic men. Int. J. Sports Med. 2003;24:419–423. doi: 10.1055/s-2003-41173. [DOI] [PubMed] [Google Scholar]
- 17.Holten M.K., Zacho M., Gaster M., Juel C., Wojtaszewski J.F.P., Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53:294–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- 18.Yan J., Dai X., Feng J., Yuan X., Li J., Yang L., Zuo P., Fang Z., Liu C., Hsue C., Zhu J., Miller J.D., Lou Q. Effect of 12-month resistance training on changes in abdominal adipose tissue and metabolic variables in patients with prediabetes: a randomized controlled trial. J. Diabetes Res. 2019;2019:1–11. doi: 10.1155/2019/8469739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuff D.J., Meneilly G.S., Martin A., Ignaszewski A., Tildesley H.D., Frohlich J.J. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–2982. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 20.Rice B., Janssen I., Hudson R., Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–691. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 21.Ismail A.D., Alkhayl F.F.A., Wilson J., Johnston L., Gill J.M.R., Gray S.R. The effect of short-duration resistance training on insulin sensitivity and muscle adaptations in overweight men. Exp. Physiol. 2019;104:540–545. doi: 10.1113/EP087435. [DOI] [PubMed] [Google Scholar]
- 22.Grøntved A., Rimm E.B., Willett W.C., Andersen L.B., Hu F.B. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch. Intern. Med. 2012;172:1306. doi: 10.1001/archinternmed.2012.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacchi E., Negri C., Targher G., Faccioli N., Lanza M., Zoppini G., Zanolin E., Schena F., Bonora E., Moghetti P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial) Hepatology. 2013;58:1287–1295. doi: 10.1002/hep.26393. [DOI] [PubMed] [Google Scholar]
- 24.Phillips M.D., Flynn M.G., McFarlin B.K., Stewart L.K., Timmerman K.L. Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Med. Sci. Sports Exerc. 2010;42 doi: 10.1249/MSS.0b013e3181b11ab7. [DOI] [PubMed] [Google Scholar]
- 25.Guedes J.M., Pieri B.L. da S., Luciano T.F., Marques S. de O., Guglielmo L.G.A., de Souza C.T. vol. 18. São Paulo); Einstein: 2019. p. eAO4784. (Muscular Resistance, Hypertrophy and Strength Training Equally Reduce Adiposity, Inflammation and Insulin Resistance in Mice with Diet-Induced Obesity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Park, Oh Lee, Kim Bae. The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. Int. J. Mol. Sci. 2019;20:4924. doi: 10.3390/ijms20194924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parry H.A., Roberts M.D., Kavazis A.N. Human skeletal muscle mitochondrial adaptations following resistance exercise training. Int. J. Sports Med. 2020;41:349–359. doi: 10.1055/a-1121-7851. [DOI] [PubMed] [Google Scholar]
- 28.Sparks L.M., Johannsen N.M., Church T.S., Earnest C.P., Moonen-Kornips E., Moro C., Hesselink M.K.C., Smith S.R., Schrauwen P. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J. Clin. Endocrinol. Metab. 2013;98:1694–1702. doi: 10.1210/jc.2012-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa J.S.R., Fonseca G.F.A.C., dos N.C., Ottone S., Silva P.A., Antonaccio R.F., Silva G., Rocha M. da S.A., Coimbra C.C., Esteves E.A., Mang Z.A., Amorim F.T., Magalhães F. de C. Strength training improves insulin resistance and differently affects mitochondria in skeletal muscle and visceral adipose tissue in high-fat fed mice. Life Sci. 2021;278 doi: 10.1016/j.lfs.2021.119639. [DOI] [PubMed] [Google Scholar]
- 30.Martin S.B., Morrow J.R., Jackson A.W., Dunn A.L. Variables related to meeting the CDC/ACSM physical activity guidelines. Med. Sci. Sports Exerc. 2000;32:2087–2092. doi: 10.1097/00005768-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 31.King A.C., Whitt-Glover M.C., Marquez D.X., Buman M.P., Napolitano M.A., Jakicic J., Fulton J.E., Tennant B.L. Physical activity promotion: highlights from the 2018 physical activity guidelines advisory committee systematic review. Med. Sci. Sports Exerc. 2019;51:1340–1353. doi: 10.1249/MSS.0000000000001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaput J.-P., Willumsen J., Bull F., Chou R., Ekelund U., Firth J., Jago R., Ortega F.B., Katzmarzyk P.T. WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: summary of the evidence. Int. J. Behav. Nutr. Phys. Activ. 2020;17:141. doi: 10.1186/s12966-020-01037-z. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bull F.C., Al-Ansari S.S., Biddle S., Borodulin K., Buman M.P., Cardon G., Carty C., Chaput J.P., Chastin S., Chou R., Dempsey P.C., Dipietro L., Ekelund U., Firth J., Friedenreich C.M., Garcia L., Gichu M., Jago R., Katzmarzyk P.T., Lambert E., Leitzmann M., Milton K., Ortega F.B., Ranasinghe C., Stamatakis E., Tiedemann A., Troiano R.P., Van Der Ploeg H.P., Wari V., Willumsen J.F. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020;54 doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colberg S.R., Sigal R.J., Yardley J.E., Riddell M.C., Dunstan D.W., Dempsey P.C., Horton E.S., Castorino K., Tate D.F. Physical activity/exercise and diabetes: a position statement of the American diabetes association. Diabetes Care. 2016;39:2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hordern M.D., Dunstan D.W., Prins J.B., Baker M.K., Singh M.A.F., Coombes J.S. Exercise prescription for patients with type 2 diabetes and pre-diabetes: a position statement from Exercise and Sport Science Australia. J. Sci. Med. Sport. 2012;15:25–31. doi: 10.1016/j.jsams.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Colberg S.R., Sigal R.J., Fernhall B., Regensteiner J.G., Blissmer B.J., Rubin R.R., Chasan-Taber L., Albright A.L., Braun B. Exercise and type 2 diabetes: the American college of sports medicine and the American diabetes association: joint position statement executive summary. Diabetes Care. 2010;33:2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heiskanen V., Hamblin M.R. Photobiomodulation: lasers: vs. light emitting diodes? Photochem. Photobiol. Sci. 2018;17:1003–1017. doi: 10.1039/c8pp00176f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anders J.J., Lanzafame R.J., Arany P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015;33:183–184. doi: 10.1089/pho.2015.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamblin M.R. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem. Photobiol. 2018;94:199–212. doi: 10.1111/php.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamblin M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4:337–361. doi: 10.3934/biophy.2017.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva G., Ferraresi C., de Almeida R.T., Motta M.L., Paixão T., Ottone V.O., Fonseca I.A., Oliveira M.X., Rocha-Vieira E., Dias-Peixoto M.F., Esteves E.A., Coimbra C.C., Amorim F.T., de Castro Magalhães F. Infrared photobiomodulation (PBM) therapy improves glucose metabolism and intracellular insulin pathway in adipose tissue of high-fat fed mice. Laser Med. Sci. 2018;33:559–571. doi: 10.1007/s10103-017-2408-2. [DOI] [PubMed] [Google Scholar]
- 42.Silva G., Ferraresi C., Almeida R.T., Motta M.L., Paixão T., Ottone V.O., Fonseca I.A., Oliveira M.X., Rocha‐Vieira E., Dias‐Peixoto M.F., Esteves E.A., Coimbra C.C., Amorim F.T., Magalhães F. de C. Insulin resistance is improved in high‐fat fed mice by photobiomodulation therapy at 630 nm. J. Biophot. 2020;13:1–16. doi: 10.1002/jbio.201960140. [DOI] [PubMed] [Google Scholar]
- 43.Gong L., Zou Z., Huang L., Guo S., Xing D. Photobiomodulation therapy decreases free fatty acid generation and release in adipocytes to ameliorate insulin resistance in type 2 diabetes. Cell. Signal. 2020;67 doi: 10.1016/j.cellsig.2019.109491. [DOI] [PubMed] [Google Scholar]
- 44.Guo S., Gong L., Shen Q., Xing D. Photobiomodulation reduces hepatic lipogenesis and enhances insulin sensitivity through activation of CaMKKβ/AMPK signaling pathway. J. Photochem. Photobiol. B Biol. 2020;213 doi: 10.1016/j.jphotobiol.2020.112075. [DOI] [PubMed] [Google Scholar]
- 45.Gong L., Zou Z., Liu L., Guo S., Xing D. Photobiomodulation therapy ameliorates hyperglycemia and insulin resistance by activating cytochrome c oxidase-mediated protein kinase B in muscle. Aging. 2021;13:10015–10033. doi: 10.18632/aging.202760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linares S.N., Beltrame T., Galdino G.A.M., Frade M.C.M., Milan-Mattos J.C., Gois M.O., Borghi-Silva A., de Biase P.F., Manchado-Gobatto F.B., Bagnato V.S., Parizotto N.A., Ferraresi C., Catai A.M. Dose response effect of photobiomodulation on hemodynamic responses and glucose levels in men with type 2 diabetes: a randomized, crossover, double-blind, sham-controlled trial. Photonics. 2022;9:481. doi: 10.3390/photonics9070481. [DOI] [Google Scholar]
- 47.F. de C. Magalhaes, C. Ferraresi, Photobiomodulation Therapy on the Treatment of Insulin Resistance: A Narrative Review, Photobiomodulation, Photomedicine, and Laser Surgery. (n.d.). [DOI] [PubMed]
- 48.da Silveira Campos R.M., Dâmaso A.R., Masquio D.C.L., Aquino A.E., Sene-Fiorese M., Duarte F.O., Tock L., Parizotto N.A., Bagnato V.S. Low-level laser therapy (LLLT) associated with aerobic plus resistance training to improve inflammatory biomarkers in obese adults. Laser Med. Sci. 2015;30:1553–1563. doi: 10.1007/s10103-015-1759-9. [DOI] [PubMed] [Google Scholar]
- 49.Duarte F.O., Sene-Fiorese M., De Aquino Junior A.E., Da Silveira Campos R.M., Masquio D.C.L., Tock L., Garcia De Oliveira Duarte A.C., Dâmaso A.R., Bagnato V.S., Parizotto N.A. Can low-level laser therapy (LLLT) associated with an aerobic plus resistance training change the cardiometabolic risk in obese women? A placebo-controlled clinical trial. J. Photochem. Photobiol. B Biol. 2015;153:103–110. doi: 10.1016/j.jphotobiol.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 50.Sene-Fiorese M., Duarte F.O., De Aquino Junior A.E., Campos R.M.D.S., Masquio D.C.L., Tock L., De Oliveira Duarte A.C.G., Dâmaso A.R., Parizotto N.A., Bagnato V.S. The potential of phototherapy to reduce body fat, insulin resistance and “metabolic inflexibility” related to obesity in women undergoing weight loss treatment. Laser Surg. Med. 2015;47:634–642. doi: 10.1002/lsm.22395. [DOI] [PubMed] [Google Scholar]
- 51.Ferraresi C., De Brito Oliveira T., De Oliveira Zafalon L., De Menezes Reiff R.B., Baldissera V., De Andrade Perez S.E., Júnior E.M., Parizotto N.A. Effects of low level laser therapy (808 nm) on physical strength training in humans. Laser Med. Sci. 2011;26:349–358. doi: 10.1007/s10103-010-0855-0. [DOI] [PubMed] [Google Scholar]
- 52.Baroni B.M., Rodrigues R., Freire B.B., Franke R. de A., Geremia J.M., Vaz M.A. Effect of low-level laser therapy on muscle adaptation to knee extensor eccentric training. Eur. J. Appl. Physiol. 2015;115:639–647. doi: 10.1007/s00421-014-3055-y. [DOI] [PubMed] [Google Scholar]
- 53.Toma R.L., Tucci H.T., Antunes H.K.M., Pedroni C.R., De Oliveira A.S., Buck I., Ferreira P.D., Vassão P.G., Renno A.C.M. Effect of 808 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in elderly women. Laser Med. Sci. 2013;28:1375–1382. doi: 10.1007/s10103-012-1246-5. [DOI] [PubMed] [Google Scholar]
- 54.Vanin A.A., Miranda E.F., Machado C.S.M., de Paiva P.R.V., Albuquerque-Pontes G.M., Casalechi H.L., de Tarso Camillo de Carvalho P., Leal-Junior E.C.P. What is the best moment to apply phototherapy when associated to a strength training program? A randomized, double-blinded, placebo-controlled trial: phototherapy in association to strength training. Laser Med. Sci. 2016;31:1555–1564. doi: 10.1007/s10103-016-2015-7. [DOI] [PubMed] [Google Scholar]
- 55.Eves N.D., Plotnikoff R.C. Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes Care. 2006;29:1933–1941. doi: 10.2337/dc05-1981. [DOI] [PubMed] [Google Scholar]
- 56.Fonseca-Junior S.J., de B C.G.A., Sá, Rodrigues P.A.F., Oliveira A.J., Fernandes-Filho J. Exercício físico e obesidade mórbida: uma revisão sistemática. ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo). 2013;26:67–73. doi: 10.1590/S0102-67202013000600015. [DOI] [PubMed] [Google Scholar]
- 57.Monroe J.C., Naugle K.M., Naugle K.E. Effect of acute bouts of volume-matched high-intensity resistance training protocols on blood glucose levels. J. Strength Condit Res. 2020;34:445–450. doi: 10.1519/JSC.0000000000002994. [DOI] [PubMed] [Google Scholar]
- 58.Yang P., Swardfager W., Fernandes D., Laredo S., Tomlinson G., Oh P.I., Thomas S. Finding the optimal volume and intensity of resistance training exercise for type 2 diabetes: the FORTE study, a randomized trial. Diabetes Res. Clin. Pract. 2017;130:98–107. doi: 10.1016/j.diabres.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 59.Daskapan A., Tuzun E.H., Eker L. Perceived barriers to physical activity in university students. J. Sports Sci. Med. 2006;5:615–620. [PMC free article] [PubMed] [Google Scholar]
- 60.Carballo-Fazanes A., Rico-Díaz J., Barcala-Furelos R., Rey E., Rodríguez-Fernández J.E., Varela-Casal C., Abelairas-Gómez C. Physical activity habits and determinants, sedentary behaviour and lifestyle in university students. Int. J. Environ. Res. Publ. Health. 2020;17:3272. doi: 10.3390/ijerph17093272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kocełak P., Chudek J., Olszanecka-Glinianowicz M. Prevalence of metabolic syndrome and insulin resistance in overweight and obese women according to the different diagnostic criteria. Minerva Endocrinol. 2012;37:247–254. [PubMed] [Google Scholar]
- 62.DeFronzo R.A., Ferrannini E., Groop L., Henry R.R., Herman W.H., Holst J.J., Hu F.B., Kahn C.R., Raz I., Shulman G.I., Simonson D.C., Testa M.A., Weiss R. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015;1 doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 63.Matsudo S., Araújo T., Matsudo V., Andrade D., Andrade E., Oliveira L.C., Braggion G., Matsudo Sandra, et al. vol. 6. Revista Brasileira de Atividade Física & Saúde; 2001. (Questinário internacional de atividade física (IPAQ): estudo de validade e reprodutibilidade no Brasil). [Google Scholar]
- 64.Warburton D.E.R., Jamnik V., Bredin S.S.D., Shephard R.J., Gledhill N. The 2020 physical activity readiness questionnaire for everyone (PAR-Q+) and electronic physical activity readiness medical examination (ePARmed-X+) Health. Fitness J. Can. 2019;12 [Google Scholar]
- 65.Riebe D., Franklin B.A., Thompson P.D., Garber C.E., Whitfield G.P., Magal M., Pescatello L.S. Updating ACSM's recommendations for exercise preparticipation health screening. Med. Sci. Sports Exerc. 2015;47:2473–2479. doi: 10.1249/MSS.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 66.Updating ACSM's recommendations for exercise preparticipation health screening. Med. Sci. Sports Exerc. 2016;48:579. doi: 10.1249/MSS.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 67.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 68.Katz A., Nambi S.S., Mather K., Baron A.D., Follmann D.A., Sullivan G., Quon M.J. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 69.Patarrão R.S., Wayne Lautt W., Paula Macedo M. Assessment of methods and indexes of insulin sensitivity, Revista Portuguesa de Endocrinologia. Diabetes Metabol. 2014;9:65–73. doi: 10.1016/j.rpedm.2013.10.004. [DOI] [Google Scholar]
- 70.Mari A., Pacini G., Murphy E., Ludvik B., Nolan J.J. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24:539–548. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 71.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 72.Cederholm J., Wibell L. Insulin release and peripheral sensitivity at the oral glucose tolerance test. Diabetes Res. Clin. Pract. 1990;10:167–175. doi: 10.1016/0168-8227(90)90040-Z. [DOI] [PubMed] [Google Scholar]
- 73.Abdul-Ghani M.A., Matsuda M., Balas B., DeFronzo R.A. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 74.de Matos M.A., Vieira D.V., Pinhal K.C., Lopes J.F., Dias-Peixoto M.F., Pauli J.R., de Castro Magalhães F., Little J.P., Rocha-Vieira E., Amorim F.T. High-intensity interval training improves markers of oxidative metabolism in skeletal muscle of individuals with obesity and insulin resistance. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malin S.K., Hinnerichs K.R., Echtenkamp B.G., Evetovich T.K., Engebretsen B.J. Effect of adiposity on insulin action after acute and chronic resistance exercise in non-diabetic women. Eur. J. Appl. Physiol. 2013;113:2933–2941. doi: 10.1007/s00421-013-2725-5. [DOI] [PubMed] [Google Scholar]
- 76.Abdul-Ghani M.A., Tripathy D., DeFronzo R.A. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139. doi: 10.2337/dc05-2179. [DOI] [PubMed] [Google Scholar]
- 77.Miyazaki Y., Akasaka H., Ohnishi T., Saitoh S., DeFronzo R.A., Shimamoto K. Differences in insulin action and secretion, plasma lipids and blood pressure levels between impaired fasting glucose and impaired glucose tolerance in Japanese subjects. Hypertens. Res. 2008;31:1357–1363. doi: 10.1291/hypres.31.1357. [DOI] [PubMed] [Google Scholar]
- 78.Gutt M., Davis C.L., Spitzer S.B., Llabre M.M., Kumar M., Czarnecki E.M., Schneiderman N., Skyler J.S., Marks J.B. Validation of the insulin sensitivity index (ISI0,120): comparison with other measures. Diabetes Res. Clin. Pract. 2000;47:177–184. doi: 10.1016/S0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 79.Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am. J. Physiol. Endocrinol. Metabol. 1987;252:E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- 80.Monzillo L.U., Hamdy O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr. Rev. 2003;61:397–412. doi: 10.1301/nr.2003.dec.397-412. [DOI] [PubMed] [Google Scholar]
- 81.Stumvoll M., Van Haeften T., Fritsche A., Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care. 2001;24:796–797. doi: 10.2337/diacare.24.4.796. [DOI] [PubMed] [Google Scholar]
- 82.McAuley K.A., Williams S.M., Mann J.I., Walker R.J., Lewis-Barned N.J., Temple L.A., Duncan A.W. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24:460–464. doi: 10.2337/diacare.24.3.460. [DOI] [PubMed] [Google Scholar]
- 83.Ismail A.D., Alkhayl F.F.A., Wilson J., Johnston L., Gill J.M.R., Gray S.R. The effect of short‐duration resistance training on insulin sensitivity and muscle adaptations in overweight men. Exp. Physiol. 2019;104:540–545. doi: 10.1113/EP087435. [DOI] [PubMed] [Google Scholar]
- 84.Kroll W. Reliability variations of strength in test-retest situations, research quarterly. Am. Assoc. Health Phys. Educ. Recreation. 1963;34:50–55. doi: 10.1080/10671188.1963.10613218. [DOI] [Google Scholar]
- 85.Levinger I., Goodman C., Hare D.L., Jerums G., Toia D., Selig S. The reliability of the 1RM strength test for untrained middle-aged individuals. J. Sci. Med. Sport. 2009;12:310–316. doi: 10.1016/j.jsams.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 86.Knight C.A., Kamen G. vol. 11. 2001. pp. 405–412. (Adaptations in Muscular Activation of the Knee Extensor Muscles with Strength Training in Young and Older Adults). [DOI] [PubMed] [Google Scholar]
- 87.Taylor J.D., Fletcher J.P. Reliability of the 8-repetition maximum test in men and women. J. Sci. Med. Sport. 2012;15:69–73. doi: 10.1016/j.jsams.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 88.Weier A.T., Pearce A.J., Kidgell D.J. Strength training reduces intracortical inhibition. Acta Physiol. 2012;206:109–119. doi: 10.1111/j.1748-1716.2012.02454.x. [DOI] [PubMed] [Google Scholar]
- 89.Willett W., Stampfer M.J. Total energy intake: implications for epidemiologic analyses. Am. J. Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 90.World Health Organization . sedentary behaviour; 2020. WHO Guidelines on Physical Activity. [Google Scholar]
- 91.Ishiguro H., Kodama S., Horikawa C., Fujihara K., Hirose A.S., Hirasawa R., Yachi Y., Ohara N., Shimano H., Hanyu O., Sone H. In search of the ideal resistance training program to improve glycemic control and its indication for patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Sports Med. 2016;46:67–77. doi: 10.1007/s40279-015-0379-7. [DOI] [PubMed] [Google Scholar]
- 92.Robertson R.J., Goss F.L., Rutkowski J., Lenz B., Dixon C., Timmer J., Frazee K., Dube J., Andreacci J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003;35:333–341. doi: 10.1249/01.MSS.0000048831.15016.2A. [DOI] [PubMed] [Google Scholar]
- 93.Lasevicius T., Ugrinowitsch C., Schoenfeld B.J., Roschel H., Tavares L.D., De Souza E.O., Laurentino G., Tricoli V. Effects of different intensities of resistance training with equated volume load on muscle strength and hypertrophy. Eur. J. Sport Sci. 2018;18:772–780. doi: 10.1080/17461391.2018.1450898. [DOI] [PubMed] [Google Scholar]
- 94.Leal-Junior E.C.P., Lopes-Martins R.Á.B., Bjordal J.M. Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: current evidence and future directions. Braz. J. Phys. Ther. 2019;23:71–75. doi: 10.1016/j.bjpt.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Francisco C. de O., Beltrame T., Hughson R.L., Milan-Mattos J.C., Ferroli-Fabricio A.M., Galvão Benze B., Ferraresi C., Parizotto N.A., Bagnato V.S., Borghi-Silva A., Porta A., Catai A.M. Effects of light-emitting diode therapy (LEDT) on cardiopulmonary and hemodynamic adjustments during aerobic exercise and glucose levels in patients with diabetes mellitus: a randomized, crossover, double-blind and placebo-controlled clinical trial. Compl. Ther. Med. 2019;42:178–183. doi: 10.1016/j.ctim.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 96.De Marchi T., Leal-Junior E.C.P., Lando K.C., Cimadon F., Vanin A.A., da Rosa D.P., Salvador M. Photobiomodulation therapy before futsal matches improves the staying time of athletes in the court and accelerates post-exercise recovery. Laser Med. Sci. 2019;34:139–148. doi: 10.1007/s10103-018-2643-1. [DOI] [PubMed] [Google Scholar]
- 97.Rossato M., Dellagrana R.A., Sakugawa R.L., Lazzari C.D., Baroni B.M., Diefenthaeler F. Time response of photobiomodulation therapy on muscular fatigue in humans. J. Strength Condit Res. 2018;32:3285–3293. doi: 10.1519/JSC.0000000000002339. [DOI] [PubMed] [Google Scholar]
- 98.Miranda E.F., Tomazoni S.S., de Paiva P.R.V., Pinto H.D., Smith D., Santos L.A., de Tarso Camillo de Carvalho P., Leal-Junior E.C.P. When is the best moment to apply photobiomodulation therapy (PBMT) when associated to a treadmill endurance-training program? A randomized, triple-blinded, placebo-controlled clinical trial. Laser Med. Sci. 2018;33:719–727. doi: 10.1007/s10103-017-2396-2. [DOI] [PubMed] [Google Scholar]
- 99.De Marchi T., Schmitt V.M., Da Silva Fabro C.D., Da Silva L.L., Sene J., Tairova O., Salvador M. Phototherapy for improvement of performance and exercise recovery: comparison of 3 commercially available devices. J. Athl. Train. 2017;52 doi: 10.4085/1062-6050-52.2.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanferdini F.J., Bini R.R., Baroni B.M., Klein K.D., Carpes F.P., Vaz M.A. Improvement of performance and reduction of fatigue with low-level laser therapy in competitive cyclists. Int. J. Sports Physiol. Perform. 2018;13:14–22. doi: 10.1123/ijspp.2016-0187. [DOI] [PubMed] [Google Scholar]
- 101.Ferreira Junior A., Kaspchak L.A.M., Bertuzzi R., Okuno N.M. Effects of light-emitting diode irradiation on time to exhaustion at maximal aerobic speed. Laser Med. Sci. 2018;33:935–939. doi: 10.1007/s10103-017-2212-z. [DOI] [PubMed] [Google Scholar]
- 102.de Oliveira A.R., Vanin A.A., Tomazoni S.S., Miranda E.F., Albuquerque-Pontes G.M., De Marchi T., dos Santos Grandinetti V., de Paiva P.R.V., Imperatori T.B.G., de Carvalho P. de T.C., Bjordal J.M., Leal-Junior E.C.P. Pre-exercise infrared photobiomodulation therapy (810 nm) in skeletal muscle performance and postexercise recovery in humans: what is the optimal power output? Photomed. Laser Surg. 2017;35:595–603. doi: 10.1089/pho.2017.4343. [DOI] [PubMed] [Google Scholar]
- 103.Dellagrana R.A., Rossato M., Sakugawa R.L., Lazzari C.D., Baroni B.M., Diefenthaeler F. Dose-response effect of photobiomodulation therapy on neuromuscular economy during submaximal running. Laser Med. Sci. 2018;33:329–336. doi: 10.1007/s10103-017-2378-4. [DOI] [PubMed] [Google Scholar]
- 104.Lanferdini F.J., Krüger R.L., Baroni B.M., Lazzari C., Figueiredo P., Reischak-Oliveira A., Vaz M.A. Low-level laser therapy improves the VO2 kinetics in competitive cyclists. Laser Med. Sci. 2018;33:453–460. doi: 10.1007/s10103-017-2347-y. [DOI] [PubMed] [Google Scholar]
- 105.Barbosa R., Marcolino A., Souza V., Bertolino G., Fonseca M., Guirro R. Effect of low-level laser therapy and strength training protocol on hand grip by dynamometry. J. Laser Med. Sci. 2017;8:112–117. doi: 10.15171/jlms.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferreira Junior A., Schamne J.C., de Moraes S.M.F., Okuno N.M. Cardiac autonomic responses and number of repetitions maximum after LED irradiation in the ipsilateral and contralateral lower limb. Laser Med. Sci. 2018;33:353–359. doi: 10.1007/s10103-017-2391-7. [DOI] [PubMed] [Google Scholar]
- 107.Dellagrana R.A., Rossato M., Sakugawa R.L., Baroni B.M., Diefenthaeler F. Photobiomodulation therapy on physiological and performance parameters during running tests: dose–response effects. J. Strength Condit Res. 2018;32:2807–2815. doi: 10.1519/JSC.0000000000002488. [DOI] [PubMed] [Google Scholar]
- 108.Toma R.L., Oliveira M.X., Renno A.C.M., Laakso E.-L. Photobiomodulation (PBM) therapy at 904 nm mitigates effects of exercise-induced skeletal muscle fatigue in young women. Laser Med. Sci. 2018;33:1197–1205. doi: 10.1007/s10103-018-2454-4. [DOI] [PubMed] [Google Scholar]
- 109.Beltrame T., Ferraresi C., Parizotto N.A., Bagnato V.S., Hughson R.L. Light-emitting diode therapy (photobiomodulation) effects on oxygen uptake and cardiac output dynamics during moderate exercise transitions: a randomized, crossover, double-blind, and placebo-controlled study. Laser Med. Sci. 2018;33:1065–1071. doi: 10.1007/s10103-018-2473-1. [DOI] [PubMed] [Google Scholar]
- 110.Mezzaroba P.V., Pessôa Filho D.M., Zagatto A.M., Machado F.A. LED session prior incremental step test enhance VO2max in running. Laser Med. Sci. 2018;33:1263–1270. doi: 10.1007/s10103-018-2475-z. [DOI] [PubMed] [Google Scholar]
- 111.Sedgwick P.M. Intention to treat analyses. Bmj. 2009;339:165–167. doi: 10.1136/bmj.b3603. [DOI] [Google Scholar]
- 112.Ferguson C.J. An effect size primer: a guide for clinicians and researchers. Prof. Psychol. Res. Pract. 2009;40:532–538. doi: 10.1037/a0015808. [DOI] [Google Scholar]
- 113.Sullivan G.M., Feinn R. Using effect size—or why the P value is not enough. J. Graduate Med. Educ. 2012;4:279–282. doi: 10.4300/jgme-d-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Magkos F., Tsekouras Y., Kavouras S.A., Mittendorfer B., Sidossis L.S. Improved insulin sensitivity after a single bout of exercise is curvilinearly related to exercise energy expenditure. Clin. Sci. 2008;114:59–64. doi: 10.1042/CS20070134. [DOI] [PubMed] [Google Scholar]
- 115.Reed M.E., Ben-Ezra V., Biggerstaff K.D., Nichols D.L. The effects of two bouts of high- and low-volume resistance exercise on glucose tolerance in normoglycemic women. J. Strength Condit Res. 2012;26:251–260. doi: 10.1519/JSC.0b013e318218dea3. [DOI] [PubMed] [Google Scholar]
- 116.Brown E.C., Franklin B.A., Regensteiner J.G., Stewart K.J. Effects of single bout resistance exercise on glucose levels, insulin action, and cardiovascular risk in type 2 diabetes: a narrative review. J. Diabetes Complicat. 2020;34 doi: 10.1016/j.jdiacomp.2020.107610. [DOI] [PubMed] [Google Scholar]
- 117.DiMenna F.J., Arad A.D. The acute vs. chronic effect of exercise on insulin sensitivity: nothing lasts forever. Cardiovasc. Endocrinol. Metab. 2021;10:149–161. doi: 10.1097/XCE.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.