Abstract
Terminalia chebula Retz, commonly known as ‘Haritaki/Myrobalan,’ has been utilised as a traditional medicine for a long time. It has been extensively exercised in various indigenous medicine practices like Unani, Tibb, Ayurveda, and Siddha to remedy human ailments such as bleeding, carminative, dysentery, liver tonic, digestive, antidiarrheal, analgesic, anthelmintic, antibacterial and helpful in skin disorders. Studies on the pharmacological effects of T. chebula and its phytoconstituents documented between January, 1996 and December, 2021 were explored using various electronic databases. During the time mentioned above, several laboratory approaches revealed the biological properties of T. chebula, including antioxidative, antiproliferative, anti-microbial, proapoptotic, anti-diabetic, anti-ageing, hepatoprotective, anti-inflammatory, and antiepileptic. It is also beneficial in glucose and lipid metabolism and prevents atherogenesis and endothelial dysfunction. Different parts of T. chebula such as fruits, seeds, galls, barks extracted with various solvent systems (aqueous, ethanol, methanol, chloroform, ethyl-acetate) revealed major bioactive compounds like chebulic acid, chebulinic acid, and chebulaginic acid, which in turn proved to have valuable pharmacological properties through broad scientific investigations. There is a common link between chebulagic acid and chebulanin with its antioxidant property, antiaging activity, antiinflammatory, antidiabetic activity, and cardioprotective activity. The actions may be through neutralizing the free radicals responsible for producing tissue damage alongside interconnecting many other diseases. The current review summarises the scientifically documented literature on pharmacological potentials and chemical compositions of T. chebula, which is expected to investigate further studies on this subject.
Keywords: Terminalia chebula, Myrobalan, Haritaki, Phytochemistry, Pharmacological activities
Terminalia chebula; Myrobalan; Haritaki; Phytochemistry; Pharmacological activities.
1. Introduction
Human civilisation and medicinal plants are long being connected to disease management. According to a World Health Organization (WHO) report, dependency on traditional medicines involving plant extracts or their active constituents is about 80% of preliminary health care needs due to the problems with current therapeutic regimens [1]. Global demands of medicinal plants to search for novel pharmacologically active compounds are increasing rigorously as they are available locally, in natural origin, with higher safety margins and lesser or no side effects. Moreover, over half of the modern drugs are derived from medicinal plants in one way or another [2]. According to the ancient references, there are approximately 500 plants that have potential therapeutic uses, and about 800 plants are being exercised in domestic medicinal practices. The Indian subcontinent is a massive depository of healthy plants utilised as conventional medicines [3]. The Combretaceae is a large family that covers herbs, shrubs and trees in globally distributed 20 genera and 600 species and has been extensively gone through scientific studies [4].
Terminalia chebula Retzius (T. chebula Retz) is a medium to large-sized tree that belongs to the Combretaceae family and is widely distributed throughout Asia. It has a few normal names like dark myrobalan, ink tree, or chebulic myrobalan (English), haritaki (Sanskrit and Bengali), Harad (Hindi), Harada (Marathi and Gujrati), Karkchettu (Telgu), and Kadukkaya (Tamil). In Tibet, T. chebula is called the “King of Medicine”. It is notable as 'haritaki' since it diverts all illnesses or is consecrated to God Shiva (Hara). Haritaki has a few fascinating equivalent words like 'pathya' since it eliminates blocks from the pathways and directs in the body; 'Abhaya', since it gives valour; 'amrita', implies ambrosia; 'Divya, implies a heavenly spice; 'media', implies a nerve tonic; 'pranada, implies life-saving; 'jivaniya', implies a vitalising spice; 'vayahstha', implies one that advances life span and keeps up with youth; 'Rasayana phala', implies a restoring organic product, and so on. In Indian folklore, this plant should be begun from the drops of ambrosia (Amrita), which fell on the earth when God Indra smashed it. Ayurvedic remedies are widely used to treat human health complications like digestive, tonic, antipyretic, spasmolytic, astringent, expectorant, antiasthmatic, antiviral, and antiviral hypoglycemic conditions [5]. It is an evergreen flowering tree, and leaves, fruits, seeds, and barks are widely used in conventional folk medicine. It is a plant with anti-bacterial [6], antifungal [7], anti-carcinogenic [8], antioxidant [9], antidiabetic [10], anti-inflammatory [11], anti-HIV [12] and anti-aging activities [13]. T. chebula is rich in bioactive compounds like tannins, flavonoids, sterols, amino acids, fructose, and resins. However, tannins like chebulinic acid, chebulagic acid, gallic acid, chebulic acid, corilagin, and ellagic acid may be responsible for the application. One-third portions of different phytoactive compounds were found [13]. This review aims to compile the recent research findings provide comprehensive and current information regarding the pharmacological potentials of T. chebula and making it a more competitive, valuable and available source of therapeutics in future drug discovery.
2. Methodology
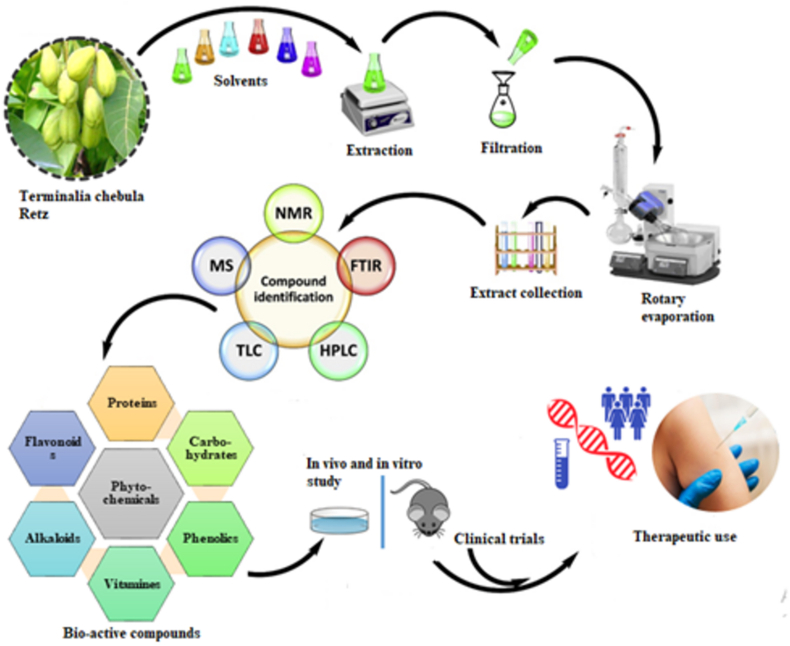
To evaluate different pharmacological perspectives of T. chebula, a comprehensive and relevant literature search strategy was conducted utilizing several authentic electronic databases, including PubMed, EMBASE, Science Direct, Web of Science, Scopus, MEDLINE, Google Scholar etc. The articles chosen were assessed based on their relevance and effective implementation. The searching keywords such as, Terminalia chebula, Myrobalan, Haritaki, Phytochemistry, Pharmacological activities etc. were used in combination with boolean words “AND” along with “OR” to carry out the literature search [14]. Recent research articles as well as some relevant supporting literature between January, 1996 and December, 2021 were chosen to retrieve information based on their appropriateness, clarity of information and conciseness. The taxonomic hierarchy, common and folk names, morphology, habitats, traditional uses of T. chebula was thoroughly documented. Positive correlation between experimental data on in vitro and in vivo assays was also done during initial article selection. The further search strategy was performed to retrieve information containing quantitative and qualitative research on pharmacological potentials, isolated chemical composition analysis as well as clinical studies of T. chebula. Articles that were retrieved went through several steps of screening procedures until the final sorting steps. An overview of the article selection process is given in Figure 1.
Figure 1.
Steps for selecting articles.
3. Introduction to plant profile
3.1. Taxonomic classification and common names
Kingdom: Plantae
- Phylum: Tracheophyta
- Class: Magnoliopsida
- Order: Myrtales
- Family: Combretaceae
- Genus: Terminalia
- Species: Terminalia chebula
3.2. Synonyms
Terminalia parviflora Thwaites, T. reticulate Roth, T. tomentella Kurz, T. aruta Buch.-Ham., ex G. Don, T. zeylannica Van Heurck & Muell. Arg.
3.3. Vernacular name
Black myrobalan, Chebulic myrobalan, Haritaki, harad, halela kabuli.
4. Morphological studies
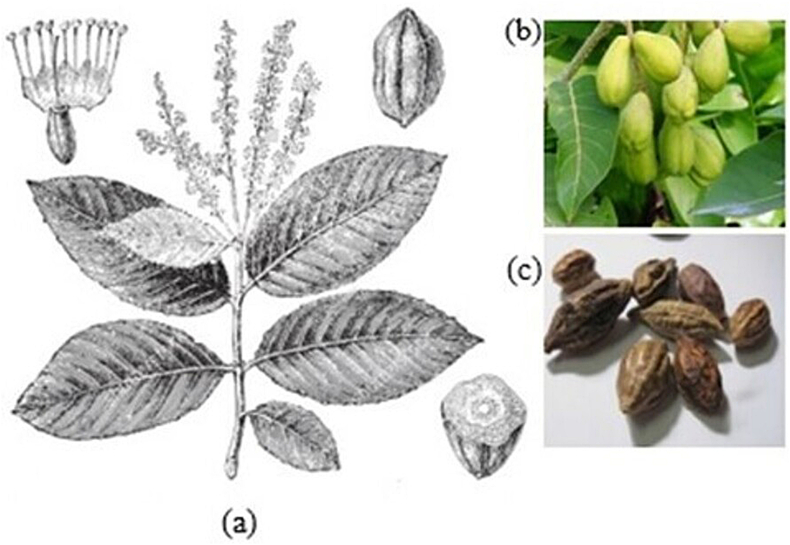
Size: T. chebula (haritaki) is an umbrella medium-sized deciduous tree up to 25–30 m in height with spreading branches. Often the bole is cylindrical and relatively short (<10 m). Leaf: bright-green, spirally arranged on the stem, 12–15 cm long and 5–6 cm wide, deciduous during the cold season. Leaf buds, branches and young leaves are like that, covered with soft, shiny reddish-brown hairs. Flowers: The flowers are short-stemmed, monoecious, dark white to yellow, with a strong, unpleasant odour, and in simple terminal spikes or short panicles. Fruit: ellipsoid drupe, dried pericarp, 2.5–4 cm long and 2–2.5 cm wide, green (unripe), brown (ripe). Depending on the location, fruit ripens from November to March, falling early after ripening. Seed: obscurely angled, rough and bony. Barks: dark brown, having a pair of large glands at the top of the petiole. The anatomical structure of T. chebula is shown in Figure 2.
Figure 2.
Anatomical structure of T. chebula (a) flower and fruit (b) unripe fruit (c) ripe fruit.
5. Natural distribution of T. chebula
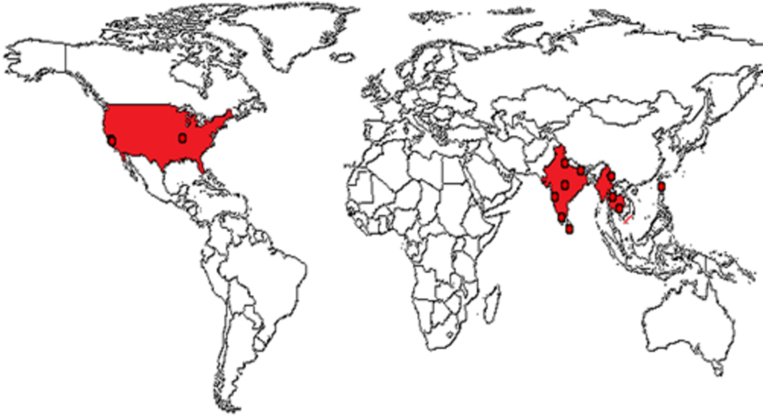
T. chebula is ubiquitous in Southeast and East Asia, specifically in Bangladesh, India, Nepal, China, Vietnam, Pakistan, Sri Lanka, Thailand, and Myanmar. The plant is well distributed in Africa, Iran, Afghanistan, and Brazil [15, 16, 17, 18]. There are 250 species of Terminalia found around the world [19]. It occurs naturally in teak, deciduous, and dry evergreen forests on free-draining clayey or sandy soils, up to 1,500 m. It is a deciduous tree having sizes from moderate to large, attains a height of 25–30m and usually has a slow growth rate [20, 21]. The tree is noted for its ability to endure both cold and drought. It is also tolerant to fire and can recover quickly after being burned [22]. A sketch of the worldwide distribution of T. chebula is drawn in Figure 3.
Figure 3.
Global distribution of T. chebula.
6. Ethnomedicinal uses
Fruits of T. chebula are stomachic, tonic, carminative, expectorant and antidysenteric. Its common uses include: rejuvenating, laxative (unripe), astringent (ripe), anthelmintic, nervine, expectorant, tonic, carminative, and appetite stimulant. It is used in people with leprosy (including skin disorders), anaemia, narcosis, piles, chronic, intermittent fever, heart disease, diarrhoea, anorexia, cough and excessive mucus secretion, and a range of other complaints and symptoms. Finely ground powder of the fruit of T. chebula is helpful to treat teeth carious, bleeding and chronic ulcer. The bark is used as a diuretic and tonic for the heart [23].
7. Phytochemistry
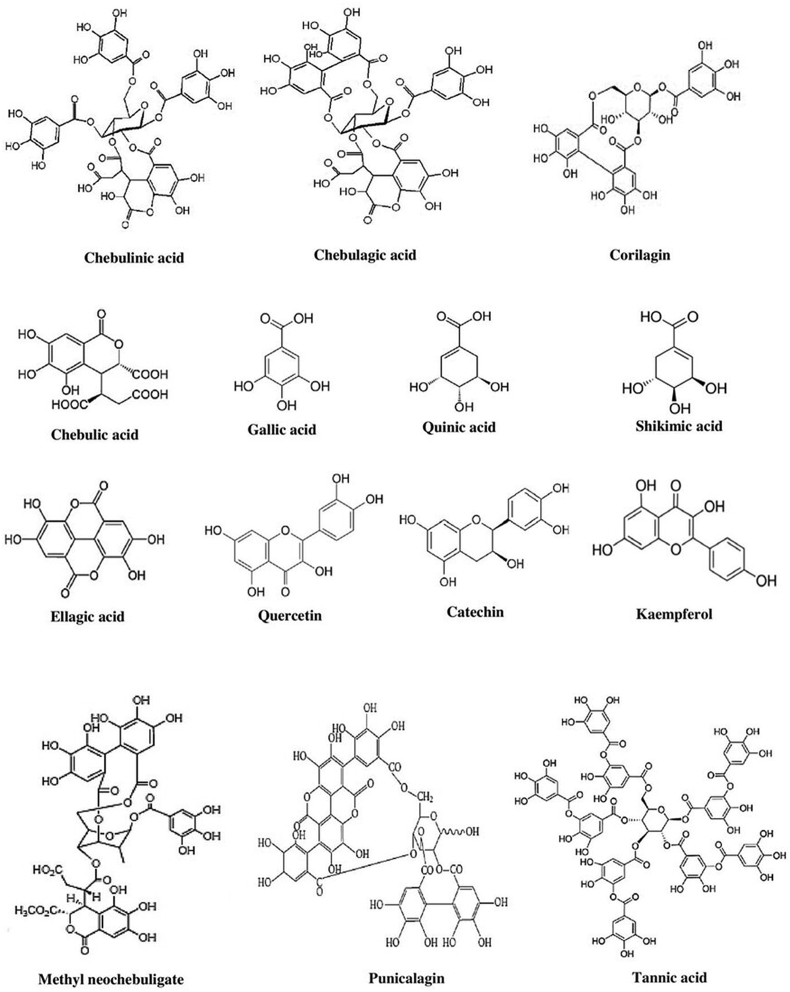
T. chebula has a plethora of bioactive compounds. According to a comparative study by Bhatt et al., T. chebula fruit contained a maximum value for total phenols, total flavonoids, gallic acid, catechin, chlorogenic acid, and coumaric acid among the ten wild edible fruits specimens tested [24]. It is likewise probably the most abundant source of ascorbic acid. The most abundant tannins in the fruit include gallic acid, ellagic acid, chebulic acid, chebulinic acid, punicalagin, terflavin A, corilagin, galloyl glucose, tannic acid. At the same time, available flavonoids like quercetin, catechin, and kaempferol are found. Saccharides like D-glucose, D-fructose, quinic acid, and shikimic acid are also present in fruit [25, 26]. A Gas Chromatography-Mass Spectrometry (GC-MS) based investigation confirmed kaempferol-3-O-rutinoside, and Vitamin E was identified in the ethyl-acetate fraction for the very first time [27]. Sterols such as β-sitosterol and daucosterol have been isolated from the fruits of T. chebula [28]. Naik et al. reported 14 essential oils in T. chebula fruits in which palmitic acid, furfural, phenylacetaldehyde, and 5-methyl furfural constitutes the major peaks [29]. Again, galls of T. chebula contain 17 bioactive compounds in two groups: 1. phenolic compounds and 2. oleanane-type triterpene acids and their glucosides [30]. Through GC-MS analysis, nine compounds were detected in T. chebula seeds with major peak areas in the 1, 2, 3-Benzenetriol (61.96%) [31]. Major phytoconstituents in T. chebula are presented in Figure 4.
Figure 4.
Structure of some major phytochemicals in T. chebula.
8. Pharmacological studies
The reported pharmacological activities of various parts of T. chebula (An overview of different pharmacological activities of T. chebula is given in Table 1) are detailed below-
Table 1.
: Overview of different pharmacological activities of T. chebula.
| Serial No | Pharmacological activities | Parts used | Responsible compounds | Extracts | References |
|---|---|---|---|---|---|
| 1 | Anti-oxidant | Fruits | Ellagic acid, ascorbic acid, gallic acid | Aqueous | [31, 32, 33, 34, 35] |
| Leaf galls | Phenolics and flavonoids | Ethanolic | [37] | ||
| Bark | Phenolics and flavonoids | Acetone | [16] | ||
| 2 | Anti-lipid peroxidation | Fruits | Casuarinin, chebulanin, Chebulinic acid and 1,6-di-O-galloyl-β-D-glucose | Aqueous | [31] |
| 3 | Anti-inflammatory and anti-arthritic | Fruits | Chebulagic acid, chebulinic acid, corilagin, hydrolysable tannins | Aqueous | [48, 49, 50, 51, 52, 53] |
| 4 | Anti-diabetic and anti-hyperglycemic | Fruits | Chebulic acid, chebuloyl group | Methanolic | [62, 63, 64] |
| Fruits | Tannin | Alcoholic | [66] | ||
| 5 | Hepatoprotective | Fruits | Chebulic acid; ellagitannins | Aqueous | [71, 72] |
| 6 | Anti-cancer | Fruits | Chebulinic acid, gallic acid, galloyl compounds | Methanolic | [81, 82, 83, 84, 85, 86, 87, 88] |
| 7 | Cardioprotective, hypolipidemic and hypocholesterolemic | Leaves, fruits, Bark | Chebulinic acid | Methanolic | [90, 91] |
| 8 | Gastroprotective and anti-ulcerogenic | Fruits | Chebulinic acid, polyphenols, flavonol aglycones | Methanolic | [97, 98, 99, 100, 101, 102, 103] |
| 9 | Neuroprotective | Fruits | Gallic acid, ellagic acid, tannic acid, chebulic acid | Methanolic | [108, 109, 110, 111, 112, 113] |
| 10 | Anti-convulsant | Fruits | Chebulinic acid, chebulagic acid | Hydroalcoholic | [118, 119] |
| 11 | Nephroprotective | Fruits, leaves | Polyphenols and tannins | Chloroform, hydroalcoholic | [61, 120] |
| 12 | Molluscicidal | Fruit | Tannic acid | Ethanolic | [126] |
| 13 | Radioprotective activity | Fruit | Polyphenols | Aqueous | [33] |
| 14 | Wound healing activity | Leaves | Hydrolysable tannins | Methanolic | [98, 136] |
| 15 | Cytoprotective activity | Fruit | Chebulic acid, gallic acid | Ethanolic | [142, 143] |
| 16 | Antiaging | Leaf galls | Polyphenols, flavonoids | Cold water | [11, 145] |
| 17 | Anti-androgenic | Fruit | Chebulinic acid | Methanolic | [147] |
| 18 | Antipsychiatry | Fruit | Chebulinic acid | Ethanolic | [149] |
| 19 | Antidiarrheal and Antimotility | Fruit | Gallic acid, 3,4,6-tri-O-galloyl- β-D-glucose, corilagin, and ellagic acid | Aqueous | [150] |
| 20 | Antibacterial | Fruit | Gallotannin and ellagic acid | Methanolic | [153] |
| Stem bark | Triterpenoids and derivatives | Methanolic | [152] | ||
| 21 | Antiviral | Fruit, bark | Punicalagin, chebulagic acid, galloyl compounds | Methanolic | [12, 161, 162, 163] |
| 22 | Antifungal | Galls, stem bark, seed | Apigenin, phytol, stigmasterol | Methanolic | [172] |
| 23 | Antiparasitic | Leaf, flower, seed | Polyphenols | Ethanolic | [175] |
8.1. Anti-oxidative properties
Reactive oxygen species (ROS) accumulation results in oxidative DNA damage, protein oxidation, lipid peroxidation and antioxidant enzyme inactivation in the cell, which lead to many chronic diseases like cancer, inflammation, diabetes mellitus, cardiovascular diseases, and atherosclerosis. Components of T. chebula (as Casuarinin, chebulanin, chebulinic acid and 1,6-di-O-galloyl-β-D-glucose) were sequestered through either chloroform, ethanolic, n-butanol or organic aqueous extracts illustrated anti-lipid peroxidation activity by decreasing the level of lipid peroxidase [32]. According to Minakshi et al., T. chebula fruit has inhibitory potential against H2O2 induced ROS generation in the THP-1 human monocytic cell line. High performance liquid chromatography (HPLC) analysis revealed gallic acid and ellagic acid, which are natural antioxidants and could be actively involved in neutralising excessive ROS production [33]. A study found that the aqueous extract of T. chebula (ellagic acid, ascorbic acid, gallic acid) was a potent inhibitor of radiation-induced lipid peroxidation and damage to the superoxide dismutase (SOD) enzyme. Owing to its entire free radical scavenging property, it can also prevent strand break formation in supercoiled DNA [34].
Polyphenols that are prominent constituents of T. chebula can donate hydrogen to tramp hydrogen peroxide scavenging activity, 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) scavenging activity, and moderately suppressed azide-induced mitochondrial ROS formation [35]. The presence of phenolics in the leaves, bark, and fruit of T. chebula has resulted in high antioxidant activity. The aqueous extract of T. chebula inhibited xanthine/xanthine oxidase activity and scavenged DPPH radicals [36]. Acetone extract pointed to hydroxybenzoic acid derivatives, hydroxycinnamic acid derivatives, flavonols, aglycon, and glycosides responsible for their antioxidative properties [17]. An antioxidant protein (∼16kDa) TCP-III from T. chebula fruit may counteract or quench ROS, reducing pro-oxidants potentiality to attack the cellular components [37]. Methanol extract of T. chebula fruit is enriched in total terpenoid content, while the aqueous extract has the total phenolic and tannin content. They represent various levels of ROS scavenging effectiveness of T. chebula fruit [33]. Also, the ethanol extract of leaf galls possessed higher total phenolics and flavonoids comprising higher antioxidant activity [38]. Another study confirmed that the methanol extract of T. chebula fruit exhibited high antioxidant activities in DPPH, ORAC, and FRAP assays [39]. The acetone extract of T. chebula bark possesses more antioxidant activity than any other extracts, with remarkable free radicals scavenging potentiality observed in acetone extract [40]. Aqueous extract of T. cheubla fruit holds back the development of age-induced damages by protecting against oxidative stress [5].
Polyherbal formulated T. chebula (Aller-7/NR-A2) was reported to inhibit free radical-induced hemolysis and lipopolysaccharide-stimulated nitric oxide release in murine macrophages [41, 42]. Alcoholic extract of T. chebula fruit which is rich in phenolic compounds have protective effects against quinolinic acid-induced oxidative PC12 and OLN-93 cells death by mitigating lipid peroxidation and intracellular ROS generation, inhibiting upregulation of nitric oxide (NO) and malondialdehyde (MDA) as well as protecting DNA from oxidative damage [43]. ‘CHL 2’ is prepared by sequential method from chloroform extract of T. chebula fruit. It displayed hydrogen donating ability and radical scavenging activity and therefore intervened in the formation of ferrous-ferrozine complex, suggesting its potential antioxidant role [44].
Various toxins and drugs may interfere with the cytochrome P-450 system to produce peroxyl and hydroxyl radicals which stimulate lipid peroxidation in the liver and cause the reduction of Glutathione (GSH) by converting to Glutathione disulfide (GSSG). T. chebula fruit extract scavenge these free radicals and inhibits GSSG oxidation, thus leading to an increase in GSH/GSSG ratio [12, 45, 46]. Interestingly, the antioxidant potential of T. chebula is somehow correlated with antiglycation activity as it inhibits the generation of advanced glycation end products (AGEs) that cause metabolic disorders like diabetes [47].
8.2. Anti-inflammatory and anti-arthritic activity
Inflammation is an organism's complex and immune response against biological, chemical, or physical stimuli. Several pathways like lipoxygenase (LOX) and cyclooxygenase (COX) are involved in inflammation and autoimmune diseases. Certain levels of pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are elevated at the onset of inflammatory response. T. chebula fruit has significant anti-inflammatory and anti-analgesic activity umpired by peripheral and central mechanisms. The mechanisms may occur due to inhibiting the synthesis of arachidonic metabolites by hydrolysable tannins and polyphenols present in fruit [48]. Furthermore, the dried fruit of T. chebula has an inhibitory effect on lipid peroxidation, a pro-inflammatory mediator. It can reduce ischemic progression by lowering TNF-α and IL-6, which indicate reversion of inflammatory response [49]. Moeslinger et al. validated the anti-inflammatory reaction of the aqueous extract of dried fruit of T. chebula by inhibiting inducible nitric oxide synthesis [50]. Nair et al. revealed the critical hindrance of joint expansion by T. chebula in contrast with formaldehyde-instigated and CFA-actuated common inflammation control.
It was also found that several pro-inflammatory cytokines like serum TNF-α level and synovial expression of tumor necrosis factor-receptor 1 (TNF-R1), IL-6, and interleukin-1β (IL-1β) were also abridged [51]. Another study reported the anti-arthritic and analgesic effects of a standardised ethanol extract of T. chebula on collagen-induced arthritis and acetic acid-induced writhing model, respectively, and the serum levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) were significantly reduced in mice [52]. Murdock et al. reported that active constituents of T. chebula (like chebulagic acid, chebulinic acid, corilagin, hydrolysable tannins) showed anti-arthritic activity in osteoarthritic dogs [53]. Tannins from T. chebula have an anti-inflammatory activity that inhibits inflammatory mediators TNF- α, reduction in beta-glucuronidase and lactate dehydrogenase enzyme, as well as inhibiting protein denaturation, and proteinase exerts anti-hyaluronidase activity in in vitro [54].
Chebulagic acid from T. chebula possesses anti-inflammatory activity by inhibiting Cyclooxygenase-2 (COX-2) and 5-Lipo-Oxygenase (5-LOX), the critical enzymes involved in inflammation [55]. The plasma membrane is central for homeostatic maintenance in mammalian cells, and loss of plasma membrane integrity is potentially involved in inflammation. Bag et al. reported that T. chebula fruit extract mimics the non-steroidal anti-inflammatory drugs (NSAIDs) in stabilising lysosomal membrane integrity [56]. Together with these, T. chebula was found to mitigate the arthritic condition by suppressing inflammatory markers TNF-R1, IL-1b, TNF-α, IL-6, MMP-3, and COX-2 granuloma formation [52, 57, 58]. AyuFlex®, a standardised formulation of T. chebula fruit, improved arthritis index in a human trial [59].
8.3. Anti-diabetic and anti-hyperglycemic activity
The global prevalence of diabetes mellitus (DM) seems to be increasing critically. Many medicinal plants with potential antidiabetic activity have been used to treat DM. The dried immature fruit of T. chebula displayed a considerable α-glucosidase inhibitory effect. It also stimulates glucose-mediated insulin secretion and intestinal glucose transport [10, 60]. Ethanol extract of T. chebula fruit significantly reduced blood glucose and glycosylated haemoglobin level and enhanced the activity of carbohydrate and glycogen metabolising enzymes. It also manifests anti-hyperglycemic activity through the upregulation of PI3-K. The chloroform extract mediates blood-glucose-lowering by amplifying insulin secretion from the β-cells of Langerhans [61]. The alcoholic extract of the fruit pulp of T. chebula has shown anti-hyperglycemic activity in albino rat models [62, 63]. The dried immature fruit of T. chebula downregulates the α-glucosidase activity or inhibits the maltase-glucoamylase complex than that of the sucrase-isomaltase complex [64, 65]. Nevertheless, there was no change in glucose transporters; thereby, inhibiting maltose hydrolysis reduces the postprandial hyperglycemic journey [66].
The methanol extract of the chebuloyl group from T. chebula non-competitively inhibits intestinal α-glucosidase, hence a potent inhibitor of postprandial hyperglycemia in managing type-2 DM [67]. Chebulic acid was efficacious in the management of the uplifted metabolic parameters, the oxidative stress by normalising the activity of G6PDH, GSH, MDA, SOD, catalysing and resorting NADPH as well as decreasing the cachectic condition along with polydipsia, polyuria and polyphagia which were commonly observed in diabetic nephropathy [68]. Treatment with ethanol extract of T. chebula diminishes the degrees of blood glucose and serum lipids, and reduce the MDA levels of serum and thoracic aorta in diabetic rodents. Notably, serum biochemical values were improved, and pathomorphological changes in the liver and kidney were observed in diabetic rodents [69].
It was previously reported that agonistic properties for both PPARα and PPARγ cause elevated glucose uptake without involvement in 3T3-L1 preadipocytes differentiation [44]. Tannin isolated from the alcoholic extract of T. chebula fruit may inhibit some carbohydrate hydrolytic enzymes such as alpha-amylase, reduce the abnormal rise in blood sugar after eating, hopefully as a promising anti-diabetic agent when compared with acarbose. Chebulic acid helps to upregulate GLUT4 and adiponectin expression, elevates C/EBP-ɑ mRNA expression, a target of PPARγ, and accelerates insulin-stimulated glucose transport in 3T3-L1 adipocytes [66].
8.4. Hepatoprotective activity
Hepatoprotective activity of T. chebula fruit extract was investigated in different animals where the hepatic damage was introduced by iron-dextran injection [70], 2-acetylaminofluorene [71], ethanol administration [72], and anti-tuberculosis drug (combination of rifampicin, isoniazid, and pyrazinamide) [73] and reported to be preventive in each case. According to Choi et al., T. chebula provides hepatic recovery by upregulating pro-inflammatory cytokines, including TNF-α, IL-1β, and anti-oxidant gene expression and protein levels in liver tissue [72]. The hepatoprotective potentials of Chebulic acid and their ellagitannins derivatives are also well-studied using rat hepatocytes in vitro [73].
During oxidative stress, several forms of freed radicals are generated, which lead to the elevation of ROS, NO, and MDA content. Aqueous extract of T. chebula (TCW) attenuates this elevated level. It provides the hepatoprotective effect to protect the hepatic tissues from oxidative injury confirmed by decreased serum biochemical parameters, including AST, ALT, and LDH [71]. TCW also leads to the upregulation in both enzymatic and gene expression of the antioxidant enzymes (SOD, CAT, GSH-Rd, and GSH-Px) to defend against oxidative damage to the hepatic tissue. Proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, which were stimulated during oxidative stress, play a role in the upregulation of iNOS protein in hepatic tissue and may lead to NO initiation free radicals, which catalyses the development of severe and acute liver injury. TCW stabilises the increased expressional level of iNOS [74]. Ethanol extract of T. chebula fruit may decrease the elevated levels of MDA lipid peroxidation to provide a hepatoprotective effect [70]. Chebulae Fructus immatures (PACF), a Tibetan traditional medicine rich in phenolic acid from T. chebula, re-establishes the liver homeostasis by upgrading the anti-oxidative defence system, alleviating inflammatory response, and hindering the hepatocyte apoptosis [75]. Phenolic acids from the immature fruit of T. chebula exhibit antioxidant activity, ease inflammation and inhibit hepatocyte apoptosis induced by CCl4 through regulation of metabolic proteins [73].
8.5. Anti-cancer activity
Chemopreventive agents can render shielding activity against the commencement of various diseases and cancer [76]. Cancer covers a broad range of sicknesses where cell growth becomes uncontrolled and then invades the lymph node, and the final step is where benign tumours become metastasised. Ethanol extract of T. chebula fruit which exhibits dual inhibitory activity in both COX-2 and 5-LOX, and so antiproliferative activity was studied on HCT-15 (colon adenocarcinoma), COLO-205 (human colon adenocarcinoma), MDA-MB-231 (breast), DU145 (prostate), and K562 (chronic myeloid leukaemia) cancer cell lines. Apoptotic mediated pathway cytotoxic activity was observed only on the COLO-205 cell line [77].
A preliminary study suggested that Ayurvedic decoction of T. chebula may possess the chemoprotective property concerning the intestine due to galloyl compounds [78]. Gallic acid (3,4,5-trihydroxy benzoic acid), a prominent component of T. chebula, showed cytotoxic potentiality and was studied to induce apoptotic cell death in various cancer cell lines, including human stomach cancer (KATO III) and human colon adenocarcinoma (COLO-205) [79]. Upon fractionation of methanol extract of T. chebula fruit was able to exhibit moderate in vitro cytotoxicity activity against human tumtumourll lines, including lung carcinoma (A-549), adenocarcinoma (SK- OV-3), malignant melanoma (SK-MEL-2), central nervous system tumor (XF498) and colon adenocarcinoma (HCT-15) [80]. Saleem et al. previously reported that extracts of T. chebula were decorated with higher phenolic contents and maybe played a role in reducing cancer cell proliferation and inhibiting lipid peroxidation. Their recent study confirms the antigrowth and antineoplastic activity of T. chebula in several cancer cell lines, including a human (MCF-7, human mammary gland adenocarcinoma) and mouse (S115) breast cancer cell line, a human osteosarcoma cell line (HOS-1), a human prostate cancer cell line (PC-3) and a non-tumorigenic, immortalised human prostate cell line (PNT1A) [19], at low concentration.
Ethanol leaf gall extract of T. chebula exhibited potent in vitro cytotoxic activity that was evaluated in several cancer cell lines, including BRL3A (buffalo rat liver), MCF-7, and A-549 cell lines [76]. Mother tincture of T. chebula decreases the survival rate of breast cancer (MDA-MB-231 and MCF-7) and non-cancerous (HEK 293) cell lines, and at extreme homoeopathic dilutions, it exhibits nanoparticulate form [81]. DNA fragmentation and shrinkage were observed when treatment was done with an aqueous extract of T. chebula on human liver cancer (HepG2) cell lines [82]. 70% methanol extract of T. chebula fruit mediates the intrinsic pathway of apoptosis in human lung cancer (A-549). In the human breast cancer (MCF-7) cell line, it settled down apoptotic activity in both extrinsic (death receptor) and intrinsic (mitochondrial) pathways as well as activates p53, thus enhancing upregulation of Bax and Bcl-2 ratio, activation of caspases leading to the cytotoxic activity in these cell lines. These polar extracts of T. chebula, which upon study on murine macrophage (RAW 264.7), was able to inhibit the production of NO, TNF- α, COX-2, and translocation of Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) from the cytoplasm to the nucleus, thus arresting inflammatory pathways that are related to the generation of various cancers [83].
Chebulinic acid, natural ellagitannins found in the fruits of T. chebula, is a potential anti-proliferative and apoptosis inducer and acts on several cancers such as breast carcinoma, colon cancer, prostate cancer, and chronic myeloid leukaemia [84, 85]. It upregulates the expression of B- cell lymphoma (Bcl-2), a critical apoptotic regulatory protein; changes the Bax and Bcl2 ratio; induces cytochrome c release; enhances mitochondrial membrane permeability; activates caspases 3; changes the Bax and Bcl2 ratio that leads to cellular death [85, 86]. Methanol extract of T. chebula shows reduction of AgNO3 to Ag-NPs where it can form intermediate colloidal complexes with phenolic (-OH) groups present in hydrolysable tannins that were observed against colon cancer and hopeful for prominent anticancer drug candidates [87]. Chebulagic acid from T. chebula squashes the Akt, ERK, JNK, and p38. The transcription factor NF-κB signal transduction pathways to downregulate multidrug-resistant mutation 1 (MDR1) mediated drug resistance in HepG2 cells through modulation of COX-2, thus amplifying the activities of doxorubicin. Chebulagic acid synergistically acts with Doxorubicin to enhance the cytotoxicity of doxorubicin to HepG2 cells by inhibiting of NF-ĸB signal pathway and down-regulating the expression of MDR1 in a COX-2-dependent manner [88].
8.6. Cardioprotective, hypolipidemic and hypo-cholesterolemic activities
Cholesterol, a fatty molecule, leads to the development of fatty streaks when deposition occurs on arterial wall intima and brings about atherosclerosis. T. chebula showed cardioprotection as it elevates serum high-density lipoprotein cholesterol (HDL-C) cholesterol levels and alleviates the serum phospholipid, cholesterol and triglyceride levels [52]. After treatment with T. chebula, liver cholesterol decreased by 50%, and cholesterol levels in the heart reduced by 60% [89]. T. chebula leaves were discovered to have a hypolipidemic effect by inhibiting cholesterol biosynthesis, enhancing plasma acyltransferase activity, and increasing faecal bile excretion activity. T. chebula also increased the activity of the enzyme lecithin-cholesterol acyltransferase (LCAT), which is involved in removing cholesterol ester that diffuses into the core of HDL particles. As a result, T. chebula may reduce cholesterol synthesis while eliminating it through bile or meta metabolising to non-harmful components. Components found from the extraction of T. chebula can be considered a promising drug candidate against cholesterol synthesis, degradation, and elimination [90].
Treatment with the methanol extract of T. chebula bark in Wistar albino mice displayed a significant reduction in the atherogenic index, total cholesterol/HDL ratio, LDL/HDL ratio, and a substantial decrease in dilatation in sinusoidal capillaries of the liver and fatty infiltration in the cytoplasm [85]. After treatment with aqueous extract of T. chebula to high cholesterol-fed rats has shown a significant reduction in aortic plaque and rich liver formation, which is assessed by a substantial increase in serum and tissue cholesterol, LDL-C, VLDL-C, triglyceride, atherogenic index, and decrease in the HDL-C levels [91]. T. chebula extract can enhance the effect of isoproterenol on the formation of lipid peroxides and retains the activity of the diagnostic marker enzyme in isoproterenol-induced myocardial injury in rats [92]. Similar findings were found in ethanol extract of T. chebula with hyperlipidemic rats in another study and thus may have defensive activity against myocardial damage [93]. Moreover, T. chebula also improves myocardial marker enzymatic markers, mitochondrial ultrastructure and energy metabolism [94]. When tested separately on normal and hyperdynamic hearts of the frogs, several extracts prepared from the T. chebula fruit showed cardiotonic activity. The extract increases cardiac output and contractility without changing the heart rate [95].
8.7. Gastroprotective effects
Traditionally, T. chebula has improved gastrointestinal motility and alleviated constipated bowel habits and comfort. T. chebula significantly decreases overall gastric lesions and gastric juice volume while increasing gastric pH and mucus release in different physical and chemical stress-induced ulcer models. Different extracts of T. chebula like chloroform extract due to the presence of tannins or hydroalcoholic extract due to the presence of carbohydrates, glycosides, triterpenoids, saponins, tannins, polyphenols, proteins, amino acids and flavonoids showed positive results against ethanol-induced gastrointestinal injury in Wistar albino rats and albino mice [96]. Gastroprotective activity of T. chebula was confirmed as chebulinic acid (CA), a significant component from its extraction elevated the mucus production level and inhibition of H+-K+ ATPase activity [97]. The antiulcerogenic action of T. chebula was balanced with a protective impact upon the gastrointestinal mucosa and the improvement in the secretory condition of Brunner's glands. It is attributed to giving protection against duodenal ulcers [98].
Another study to assess the potential antiulcerogenic effect of T. chebula, methanol fruit extract on animal models was explored, which resulted in the significant inhibitory action of gastric ulceration through degeneration, haemorrhage, and edematous appearance of the gastric tissue [99]. An investigation revealed that CA derived from T. chebula notably possesses gastroprotective and antisecretory action [100]. Due to the presence of chebulagic acid and polyphenols, it was found that T. chebula upregulates the expression of VEGF/MMP-2 and alleviates the healing of ethanol-induced gastric ulceration [101]. Helicobacter pylori are highly adaptive to the human stomach, with many unique characteristics that allow it to enter mucus, directed swimming and multiplication in mucus, attached to the epithelium cells, escape immune responses, and as a result, persistent colonisation and transmission. Treatment with T. chebula either may eradicate H. pylori or increase the secretory status of the Brunner's gland, or by inhibiting its attachment by increasing surface hydrophobicity or upregulating mucin secretion, and inhibiting proton pump or solid inhibitory activity against IL-8 secretion, suggesting gastrointestinal protection against H. pylori [102, 103].
Triphala formulation containing T. chebula, an Indian herbal drug, exerts a more effective protection against methotrexate-induced damage in rat intestines [104]. Although the active constituents that are responsible for the antiulcer property was not proved through this study, flavonoid compounds such as hydroxybenzoic acid derivatives, flavonol aglycones and glycosides of the plant may be involved since flavonoids have been documented to retain remarkable antiulcer properties through many ulcer-instigated methods [105, 106]. Additionally, ethanol extract of T. chebula recorded the gastroprotective and antiulcer properties of the plant. The increasing doses upon ethanol-instigated models prominently reduced total acidity, free acidity, and ulcer index levels which upregulated the mucin and pepsin secretion and improved the protection percentage [107].
8.8. Neuroprotective effects
Methanol and aqueous extracts of T. chebula, due to the presence of ellagic acid, exhibit favourable neuroprotective activities against H2O2-induced toxicity and Aβ25-35 toxicity to pheochromocytoma (PC12) cells and are potential candidates for the treatment of neurodegenerative diseases caused by H2O2 and Aβ25-35. In addition, the intracellular calcium levels, which were depleted by H2O2 and Aβ25-35 induced in PC12 cells, are modulated by the presence of ellagic acid [108]. Earlier studies have reported that gallic acid, ellagic acid, and tannic acid have exhibited antagonistic effects on both acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) in a dose-dependent manner [109, 110]. Some studies have reported that different extracts of T. chebula exhibited AChE inhibitory activity and NMDA receptor antagonist [111, 112]. Recent studies have confirmed that methanol extract of T. chebula shows potent AChE, BChE, antioxidant and root elimination inhibitory activities compared to standard drugs due to 7-methyl gallic acid. 7-methyl gallic acid acts as a multifunctional agent with dual efficacy cholinergic activity, which exhibited anti-amyloidogenic effects by effectively inhibiting the formation of Aβ-filaments and destabilising mature preformed filaments. These results are a multipotent neuroprotective effect that can be considered for treating AD and associated neurodegenerative disorders [113].
Quinolinic acid pathological accumulation has been shown to induce neuroinflammatory and demyelinating diseases such as multiple sclerosis by overproducing free radicals. Alcoholic extract of T. chebula, rich in phenolic compounds, reduces lipid peroxidation and free oxygen radical generation. This work offers new therapeutic approaches for T. chebula in protecting oligodendrocytes against QA-induced toxicity in neuronal cells; hence, it can be used for neurodegenerative and demyelinating diseases that were studied on PC12 and OLN93 cell lines [43]. Treatment with seed extract maintained elevated immunoreactivity as well as relative protein levels for Zn-superoxide dismutase (SOD1), Mn-superoxide dismutase (SOD2), and brain-derived neurotrophic factor (BDNF) in the hippocampal C1 region compared with ischemic damage (vehicle) group in male Mongolian gerbils after four days of ischemia-reperfusion [114]. Acute administration of T. chebula ethanol extracts improves learning and memory recall by suppressing oxidative stress and apoptosis in mice in an inverse dose-dependent manner [115].
Hydroalcoholic tannin-rich section of T. chebula down-regulated serum cortisol levels, but there was an upregulated expression of BDNF, cAMP response element-binding protein (CREB), gamma-aminobutyric acid A receptor (GABAA), and 5-Hydroxytryptamine 1A receptor (5-HT1A). Anxiety was minimised due to the elevated level of neurotransmitters, namely 5-HT (serotonin), DA (dopamine) and NE (nor-epinephrine) [116]. Chebulic acid significantly rescued neuronal cells from the cytotoxicity induced by MPP+, the active neurotoxic metabolite converted from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) by monoamine oxidase-B (MAO-B) in astrocytes, is the proximal neurotoxin destroying the nigrostriatal pathway and is hopeful for a protective key functioning role in Parkinson's disease-related damage [117].
8.9. Anticonvulsant activity
T. chebula has been traditionally practised to manage epilepsy and other CNS disorders in the Ayurvedic system. Debnath et al. performed a study on Swiss albino mice administering an ethanol extract of T. chebula at inat00 mg/kg and 500 mg/kg, which conferred anticonvulsant properties. It was found that T. chebula fruit extract provided a protective effect to the experimental models from seizures induced by the maximal electrical shock (MES) as well as slowed down the latency of pentylenetetrazole (PTZ) and picrotoxin (PC) instigated seizures, possibly with the help of the opening of chloride channel on GABA receptors [118].
Hydroalcoholic fruit extract of T. chebula (HETC) exhibits significant anticonvulsant activity and also increases the sub-therapeutic dose of phenytoin and valproate. At 1,000 mg/kg doses, T. chebula fruit rendered 83.33% and 66.66% protection on experimental animals from seizures, and seizures-induced cognitive impairment produced by MES and PTZ, respectively. On the other hand, the standard dose of valproate gave a 66% similar protective effect. However, a combination dose of T. chebula fruit extract (1000 mg/kg) with valproate and phenytoin (150 mg/kg) conferred complete protection and suggested its utility as an adjunct to antiepileptic drugs to prevent cognitive decline and oxidative stress [119]. Ethanol extract of T. chebula reduces seizures' duration and may delay the latency of attack, hopeful for benefits of anticonvulsant and proconvulsant activity [118]. These investigations pharmacologically justified the usage of T. chebula in treating epilepsy as traditional medicine in India [21].
8.10. Nephroprotective effects
Cisplatin is commonly used as a therapeutic target to treat multiple tumour forms, but the primary side-effect of this chemotherapeutic agent is nephrotoxicity. Hydroalcoholic extract of T. chebula contains various phytoconstituents, which hold strong antioxidant properties and mitigate inflammation, ROS generation, tubular necrosis, cell apoptosis, and mitochondrial dysfunctioning initiated by cisplatin. The increased serum TNF-α levels confirmed this; typical architecture of the cortex and proximal tubular cells, no inflammatory infiltration, decreased level of MDA, and debilitated depletion of antioxidant defence system (GSH and SOD levels) were studied on normal rats [120]. In vitro study of T. chebula on NRK-52E and MDCK kidney epithelial cells were antilithiatic against oxalate-induced urolithiasis [121]. Moreover, animals treated with T. chebula extract caused a significant reduction in serum level of triglycerides, total cholesterol, creatinine, aspartate transaminase, and blood urea nitrogen compared with the acetaminophen (APAP) induced nephrotoxic control [122]. Gentamicin is an aminoglycoside antibiotic used in Gram-negative infections. T. chebula in Bilvadi agada, a polyherbal formulation, used as antivenom in the management of low potent poisons, which maintains alkalinity at tubular levels, inhibits the accumulation of Gentamicin in lysosomes and thus prevents phospholipidosis and renal damage that was assessed by significant changes only in the levels of serum creatinine, urine creatinine, and urine potassium showing a protective effect of the incidence [115].
Heavy metal induces nephrotoxicity, a typical incidence of acute kidney injury (AKI). Among them, mercuric chloride, highly poisonous and toxic metals that exist friendly in the industrial area to our environment, shows a great affinity towards proximal convoluted tubule (PCT), facilitates the efflux of calcium particles from kidney mitochondria and debilitates the cellular function, repress the activity of antioxidant enzymes, upregulate TNF-α production, gathered there and making an insult to the nephron. Aqueous extract of T. chebula decreases MDA levels. It increases the activity of GSH, SOD, and GPx, which inhibits mercury gathering and makes a straightforward way for the nephron hopeful for a promising candidate against mercury-induced toxicity [123].
Chloroform extracts of T. chebula have inhibited the formation of AGEs and impeded the stated pathway, providing a significant renoprotective activity [61]. Treatment with hydroalcoholic fruit extract of T. chebula attenuated apoptosis by decreasing the expression of Bax, caspase-3, and MDA levels and increasing the expression of Bcl-2 in renal tubules, which was checked out by a reduced level of serum creatinine, BUN, and MDA levels. T. chebula also inhibited N-diethyl nitrosamine-induced renal carcinogenesis, reducing the number and percent of tumours in rats with renal cell tumours [124]. Doxorubicin is an anthracycline anticancer drug used to treat various cancers for a long time but is limited in its use due to different types of toxicity arising with it. Treatment with T. chebula decreased the renal complications associated with doxorubicin administration, and the possible mechanisms include wound healing and antimicrobial and antioxidant properties [125].
8.11. Molluscicidal activity
HPLC exhibited that T. chebula fruit contains tannic acid as the essential compound, which is more soluble in ethanol than other solvents [126]. The ethanol extract of the T. chebula powder induces time and concentration-dependent toxic effects against Lymnaea acuminata, tannic acid working as a potential molluscicide [127]. L. acuminata is a freshwater snail that acts as an intermediate host of liver fluke Fasciola gigantica, causing fasciolosis/schistosomiasis disease in humans and livestock [128]. Furthermore, Upadhyay and Singh, through in vitro and in vivo studies, assessed that tannic acid of T. chebula fruit in sublethal dose could activate inhibition in a competitive-non-competitive manner against acetylcholinesterase (AchE), acid phosphatase (ACP), and alkaline phosphatase (ALP) present in the nervous tissue of L. acuminata [129]. Hence, plant-derived molluscicides can be safer, cheaper and more feasible for non-target animals than synthetic ones [130, 131].
8.12. Radio-protective effect
Aqueous extract of T. chebula possesses various phytoconstituents that could protect cellular organelles from the radiation-induced damage and prevent strand break formation on DNA, which initiates by γ–radiation on cancer treatment. Hence it may be considered to have a radio-protective ability [34]. Dixit et al. found the fruits of T. chebula against γ-irradiation-mediated oxidative stress in rats. The result showed that prior supplementation of T. chebula extracts caused a significant increase in the colony-forming unit (CFU) in the endogenous spleen, decreased radiation-induced cellular DNA damage in peripheral rat blood and reduced intestinal cell death [132]. The ability of T. chebula extract to protect the DNA integrity against radiation was also investigated where the original supercoiled formation of bacterial plasmid DNA (pBR322) was well maintained and thus, preventing strand breaks and necks formation [34, 133].
8.13. Wound healing property
The wound healing property of T. chebula was reported in granulation tissues [98]. T. chebula extracts markedly elevated total protein, matrix molecules such as hexosamine and uronic acid, intact DNA and collagen (90%) in the epithelialisation period and increased tensile strength. Nasiri et al. reported the wound healing property of T. chebula herbal preparations as it hastened the recovery from burn injury [134]. T. chebula Fructus water extract demonstrated significant healing efficacy against vascular smooth muscle cells (VSMC) wound in a dose-dependent fashion [135]. As an extract of T. chebula has potent antioxidant properties, it can prevent lipid peroxidation and promote the healing process. Topical administration of T. chebula enhanced the rate of wound contraction and collagen turnover and shortened the period of epithelialisation, which supports wound healing effectiveness. T. chebula treated incision wound tissues exhibited higher tensile strength by about 40%, indicating a more significant collagen concentration at the wound site. T. chebula counters postoperative wound infection, a potent inhibitor of Staphylococcus aureus and Klebsiella, organisms mainly responsible for postoperative infection [98, 136].
Tannin-rich extract from immature fruits accelerated granulation tissue formation and collagen organisation with a significant rise in VEGF in rats with cutaneous wounds [18]. Again, treatment with fruit extracts maintained a higher 3D extra-cellular matrix and metabolic activity due to high actin and keratin expression in fibroblast and keratinocytes. Thus, believe in wound healing and facilitating complete dermal repair [137]. Wound healing became a challenging issue in diabetic people, which can be attenuated after applying T. chebula. Methanol extract from leaves of T. chebula increases the rate of angiogenesis. It improves antioxidant enzymes status, and the seeds of T. chebula significantly increased the level of superoxide dismutase and nitric oxide and decreased lipid peroxidation in granuloma tissue, leading to faster wound healing activity (by 83% compared to the commercial ointment) observed in diabetic mice [138].
8.14. Cytoprotective activities
It is well known that UV irradiation produces DNA strand breaks, DNA-protein crosslinks and base modifications mediated by ROS. It can result in skin cancer, inflammation and photoaging. In HEK-N/F cells, T. chebula ethanol extract reduced oxidative stress and manifested a cytoprotective effect against UVB-induced damage [139]. In addition, Yakaew et al., by using skin fibroblasts and mice model, reported that the ethanol extract of fruits reduced the UVB-instigated MMP-1 (matrix metalloproteinases-1) as well as MMP-13 (matrix metalloproteinases-13) expression together with the upregulation in the face of type 1 procollagen in UVB, radiated human skin fibroblasts. It suggested that the preventive effect of T. chebula was antagonistic towards dermal photodamage [140]. Using a human umbilical vein endothelial cells model, chebulic acid present in the T. chebula exerts inhibitory action upon AGEs-induced dysfunction of endothelial cells [141].
T. chebula fruit extract-derived chebulic acid, and gallic acid was documented to exhibit cytoprotective and immunosuppressive properties and impede the cytotoxic T lymphocyte (CTL)-mediated cytotoxicity, including cytotoxicity granule exocytosis that responded by anti-CD3 stimulation [142, 143]. CA extracted from T. chebula significantly reduced free acidity (48.82%), total acidity (38.29%), and upregulated mucin secretion by 59.75%, respectively, at a dose-dependent study. Further, CA significantly inhibited H+ K+-ATPase activity in an in vitro study confirming its anti-secretory activity. It also provided cytoprotective training by inhibiting the formation of lesions through inhibition of acid secretion that was studied in rat models [97]. Extract of T. chebula also showed cytoprotective properties towards the MDCK and NRK-52E cells by reducing the LDH leakage and increasing the cell viability in urolithiasis patients [121]. The healing activities of T. chebula were moderate (protection of around 43%) on its ethanol extract against indomethacin-induced stomach ulceration [144].
8.15. Antiaging activities
Stress causes dysregulation in the immune cells and speeds up the ageing process. Plasma levels of adrenaline and noradrenaline are enhanced during stress induced by the swimming endurance test. The swim endurance test results indicate that the T. chebula extract has the properties that increase physical endurance and maintain the average plasma level of catecholamine and MAO. It boosts muscle glycogen levels, decreases the concentrations of muscle lactic acid and ammonia (two toxic by-products of muscular effort) in rats, and possesses significant antioxidant activity. Hence, it provides substantial stress-induced antiaging exercise [145].
Coldwater extract of T. chebula gall at a dose of 0.1 mg/mL exhibited a significant chelating and tyrosinase inhibition activity and the highest DPPH radical scavenging activity with an increased level of antioxidant enzymes compared to other medicinal plants. Coldwater extract of T. chebula gall indicated the highest stimulation index (SI) on normal human fibroblast proliferation of 1.441, which was more active than ascorbic acid (SI 1.21). This extract has also demonstrated MMP-2 inhibition on fibroblasts determined by zymography, and it was 1.37 times more potent than ascorbic acid, considering its development as an antiaging product [11].
8.16. Anti-spermatogenic activity
A recent study documented the involvement of this plant having anti-androgenic properties through decrement in the length of germinal epithelium, germ cell number, reproductive organs weight, and diameter in stage VII tubules. Therefore, it may benefit from developing male contraceptives [146]. Moreover, it was found that a polyherbal preparation containing T. chebula extract has exerted reduced testicular action by alleviating the level of androgenic and semiological sensors in experimental models [147].
8.17. Antipsychiatry activity
Treatment of ethanol extract of T. chebula at 100 or 200 mg/kg, p.o dose levels showed significant improvement in the duration of immobility time in depressant models like forced swimming test (FST), tail suspension test (TST). Moreover, the anxiolytic activity of the same extract treatment was evidenced by a significant increase in the duration of time spent in the open arm and the number of head dips in the elevated plus-maze model. The results showed that T. chebula fruits could be an alternative for treating numerous central nervous system disorders such as antidepressants and anxiety [148]. CA, found in the extracts of T. chebula, exhibited p < 0.01 (in FST) and p < 0.05 (in TST) independent of dose when investigated for antidepressant activity. However, only 20 mg/kg and 40 mg/kg showed significant anxiolytic potentials in the elevated plus-maze, light-dark box test, and hole-board paradigms compared with the control (P < 0.05). The results suggest antidepressant and anxiolytic potentials of CA [149].
8.18. Antidiarrheal and antimotility activity
Aqueous extract of T. chebula fruit possesses significant antidiarrheal activity, inhibiting gastrointestinal propulsion and fluid secretion. The ethyl acetate fraction, the most active fraction of Chebulae Fructus, mainly contains gallic acid, 3,4,6-tri-O-galloyl- β-D-glucose, corilagin, and ellagic acid, which decreases the frequency of stooling [150]. Alcoholic and aqueous extract of T. chebula reduced intestinal transit time (anticholinergic effect) and reduced gastric emptying. The presence of tannins, alkaloids, saponins, flavonoids, sterols and triterpenes also supports its antidiarrheal and antimotility activity [150].
8.19. Antibacterial activity
The potential anti-bacterial activity of T. chebula has been investigated in several studies. Antibacterial activity was found in T. chebula against both gram-positive and gram-negative human pathogenic microorganisms [36]. Leaf extract is bactericidal against Escherichia coli, Shigella sp, Vibrio cholerae and Salmonella sp [151]. It also has inhibitory activity on-ear pathogens [152]. Triterpenoids and their derivatives were isolated from the stem bark of T. chebula that showed potential antimicrobial on Bacillus subtilis, S. aureus, Salmonella typhi, Pseudomonas aeruginosa and E. coli [6]. Gallotannin from the fruit of T. chebula works synergistically with gentamicin and trimethoprim as an efflux pump inhibitors. Bacteria can make multidrug resistance by efflux pump mechanism [153]. Ellagic acid and its structural derivatives from T. chebula reduce the virulence of P. aeruginosa via downregulation of phosphate kinase-1 (PPK-1), leading to the reduction of RpoS expression, which is responsible for the transcription of survival genes in microorganisms [154].
Methyl gallate isolated from T. chebula showed its antimicrobial activity against Shigella sp, causing extracellular and intercellular disintegration of Shigella dysenteries, leading to cytoplasmic content leakage to kill the pathogen [155]. It functions against Helicobactor pyroli, a common bacterium that causes stomach cancer, ulcers, and gastritis, by decreasing urease activity. When exposed to the T. chebula extract, the methicillin-resistant S. aureus displayed decreased growth and activity, demonstrating the antibacterial activity. The ripe seeds have shown potent anti-bacterial properties that are particularly effective against S. aureus. Salivary bacteria called Streptococcus mutants were severely constrained in their growth by the aqueous extract of T. chebula [36].
8.20. Antiviral activity
Several studies have documented the restraining property of T. chebula viral diseases generated by herpes simplex virus-1 (HSV-1), cytomegalovirus (CMV), influenza A, and human immunodeficiency virus type 1 (HIV-1) [120,[156], [157], [158], [159]]. Ahn et al. reportedly found that T. chebula fruits comprised four HIV-1 integrase inhibitors, namely gallic acid and three galloyl glucose. Here, galloyl compounds perform a pivotal role in blocking the 3′- processing of HIV-1 integrase [12]. T. chebula is also attributed to possessing a significant property of inhibiting retroviral reverse transcriptase [160]. The fruit extract of T. chebula displayed a suppressive action against HIV-1 reverse transcriptase [161]. Kurokawa et al. performed an investigation that revealed this plant's ability to elicit a more potent antiviral effect on HSV-1 infection in combination with acyclovir via in vivo and in vitro studies in mice models [157].
This plant is also used traditionally in acute respiratory infection, which hinders the cytopathic activity of the influenza A virus to protect the epithelial cells [162]. Two hydrolysable tannins were obtained from the dried fruits of T. chebula, such as punicalagin (PUG) and chebulagic acid, which rendered an inhibitory effect on the entry of HSV-1 in A549 through targeting and disabling HSV-1 viral components. They also attenuate lesions and lessen viral loads and inflammatory markers (iNOS, COX-2, PGE2) in BALB/c rat lungs infected with respiratory syncytial virus [163]. The mechanism that confers the broad-spectrum antiviral activity against respiratory syncytial virus infection involves the downregulation of the IKK-NF-κB and MAPK signaling pathways. Again, chebulagic acid suppressed viral replication and cytopathic effect in enterovirus 71 infected rhabdomyosarcoma cells [164]. Through investigation of the immunosuppressed mice model, Yukawa et al. evaluated that aqueous extract of T. chebula markedly restrained the viral replication process of human CMV [159].
A recent study explored that chebulinic and chebulagic acid derived from T. chebula produce straightforward antiviral action and efficiency against HSV-2 through inhibition of viral attachment and penetration into the host cells in comparison to acyclovir, a standard medication employed for the management of HSV infection [165]. Besides, T. chebula was investigated for its potentiality against AIDS and other sexually transmitted diseases [166]. The aqueous extract of T. chebula was found to perform a pivotal function against hepatitis B virus (HBV), employing decrementing the number of extracellular HBV virion DNA. It may pave the way for its potential usage as a competent anti-HBV drug in the future [167]. As a newly developed alternative drug, the acetone extract of T. chebula lately can be used to manage swine influenza A infection due to its simple preparation method, potent action, and cost-effectiveness [168]. The methanol bark extract of T. chebula showed antiviral activity regarding inhibitory effects on syncytium formation and cell surface expression of viral glycoprotein in BHK cells infection caused by Newcastle disease virus (NDV) [169].
8.21. Antifungal activity
Galls of T. chebula inhibited all the three Trichophyton spp. (Trichophyton mentagrophytes, T. rubrum, T. soudanense) And three Candida spp. (Candida albicans, Torulopsis glabrata, and C. krusei) At the same time, T. chebula seed extract exhibited an inhibitory effect only on T. glabrata [7]. Seed of T. chebula also showed fungicidal potency and adverse effect on spore germination against plant fungi named Fusarium oxysporum, Fusarium solani, Phytophthora capsici and Botrytis cinerea in a concentration and time-dependent kinetics [170]. Apigenin, a natural flavone from the stem of T. chebula found to be anti-fungal against T. mentagrophytes induced dermatophytosis in mice [171]. Phytol and stigmasterol isolated from the stem bark of T. chebula showed inhibitory effect on Candida arrizae, Aspergillus niger, C. albicans and Saccharomyces cerevisiae [172].
8.22. Antiparasitic activity
T. chebula was reported to possess anthelmintic against Haemonchus contortus and antiplasmodial activity [173, 174]. Leaf, flower, and the seed of T. chebula have parasite lethality against blood-sucking parasites like Rhipicephalus microplus, Damalinia caprae nymph, Haemaphysalis bispinosa and Paramphistomum cervi [175]. Following the Ayurvedic system, a polyherbal preparation containing T. chebula has displayed a marked and potent activity compared to Albendazole on earthworm Pheritima Posthuma by increasing the concentration of extracts [176].
T. chebula ethanol extract acts as a natural anthelmintic that has produced inhibitory effects on the action of AChE by blocking AChE activity and also decreases the motor activity of Cotylphoron cotylphorum in a time-dependent concentration manner. Halting the motility of worms and a repercussion from the intestinal peristalsis of the host also occurred [177]. Chitosan covered AuNPs of T. chebula increased the influx of ROS and are believed to lead to apoptosis and mitochondrial dysfunction in human filarial parasite W. bancrofti and bovine filarial parasite S. cervi through membrane phosphatidylserine out shifting, elevated expression of Nrf2 and its target proteins, increased GST and reduced GSH activity [178].
9. Clinical studies and safety evaluation of T. chebula
T. chebula is one of the most versatile plants with a broad spectrum of medicinal and pharmacological properties. Various investigations alluded that T. chebula can play a justifiable role as a safe drug candidate because of its remarkable cardioprotective, antioxidant, cytoprotective, chemoprotective, and hepatoprotective properties. As T. chebula manifests no enfeebling or toxic effects, it can be developed as a nutraceutical formulation or herbal functional sustenance in future [120]. A clinical trial was performed, which showed that T. chebula has an effect on evacuating the bowel entirely in patients suffering from constipation [179]. Oral rinsing with T. chebula extract demonstrated a remarkable decrement in both total bacterial count and Streptococcal count in saliva samples. After rinsing, T. chebula gave a defensive effect by preventing dental cagbtries for up to 3 h [23]. A controlled clinical study consisting of seventy-eight patients reported the substantive property of 10% T. chebula extract in comparison to 0.12% chlorhexidine mouth rinse to cure gingivitis and dental plaque. In this investigation, the 10% mouth rinse of T. chebula extract was found to be beneficial in neutralizing salivary pH as well as gingival irritation and microbial plaque, while there was no statistical difference in reducing clinical parameters between groups of chlorhexidine and T. chebula [180].
In addition, various trials found that some Ayurvedic formulations containing T. chebula rendered potential action upon constipation, mental stress, physical and mental disability, and allergic rhinitis. Without displaying any adverse reactions, herbal drugs consisting of T. chebula provided positive effects in the treated experimental subjects in comparison to normal control groups [181, 182, 183, 184]. Histopathological tests revealed when T. chebula pretreated animals were subjected to isoproterenol showed mild oedema, inflammatory cells with a reduction in density, and no concurrent area of multiple sub-endocardial damages compared to the controls [92]. According to a human trial, T. chebula may play a vital role in treating hemorrhoids in patients who were administered T. chebula supplements. It showed a significant variation between the test group and the control group in declining hemorrhoid mass size as well as constipation [182].
In order to investigate the safety and effectiveness of analgesic activity of T. chebula, a double-blinded, placebo-controlled clinical trial was conducted recently on 12 healthy subjects who were randomly given either 1000mg of T. chebula capsules or a placebo. In order to generate a thermal pain sensation, a hot air analgesiometer was employed for measuring thermal pain, wherein the test reported a notable elevation in mean pain threshold, the mean percentage change in pain threshold (20.42%), and mean tolerance time together with the mean percentage change in tolerance time (17.50%) in subjects treated with T. chebula capsules compared to placebo and baseline. The study recommends the T. chebula capsule as a treatment option for chronic pain, particularly in geriatric patients who are at high risk of gastrointestinal and renal problems [185]. Moreover, another clinical trial has also evaluated the analgesic property of T. chebula in healthy subjects, which employed an analgesy meter incorporating a mechanical pain approach to show a substantial amelioration in mean pain threshold and tolerance force as well as time without any adverse effects [186]. At a dose of 140 mg/kg, T. chebula notably increased gastric emptying in models against cisplatin and instigated changes in gastric motility and intestinal transit [187].
A recently published clinical study has been aimed at evaluating the effectiveness of aqueous extract of T. chebula fruit (AyuFlex®) on joint discomfort, mobility, and overall joint capacity in healthy overweight adults. At doses of 250mg and 500mg twice a day, the T. chebula extract was administered for 84 days, which helped to improve modified-Knee Injury & Osteoarthritis Outcomes Score (mKOOS) of the knee and joint mobility, as well as total joint health in mostly healthy populations with exercise/activity-induced knee discomfort, including knee soreness. The findings of the trial imply that the advantages may further increase from knee health to total joint and spine health [60].
The adverse and toxic effects of herbal drugs are mostly linked to hepatotoxicity. Besides, many studies also documented other adverse effects, such as toxicity of the nervous system, kidney, hematopoietic and cardiovascular system, carcinogenicity, and mutagenic effects. Before the efficacy test, various preliminary biological tests were performed to ensure the herbal medicines' safeness [162]. A safety assay investigated the toxic effects of T. chebula via in vitro and in vivo along with acute and chronic toxicity tests, which found no adverse reactions and changes in biochemical, morphological and parameters caused by T. chebula (up to 1000 mg/kg body weight dose). Besides, it is documented that T. chebula is free from mutagenicity, cytotoxicity, genotoxicity, and up to a certain level of dosage [188].
9.1. Non-cytotoxic effect
Ahmad et al. evaluated an experiment on cellular toxicity of T. chebula in which the crude extract of the plant (concentrated in aqueous, ethanol and ethyl acetate) did not exhibit any toxic cellular effects on sheep erythrocytes as well as acute oral toxicity in rats at recommended or higher doses [189, 190, 191]. In addition, it was found that T. chebula fruits had no cytotoxic action on the Allium model [192].
9.2. Non-genotoxic effect
The VITOTOX test and Ames assay evaluated that T. chebula extracts contain no genotoxic effects [193]. Another investigation suggested that T. chebula significantly decreased genotoxicity produced by lead and aluminium [194, 195]. T. chebula containing hydrolysable tannins induced inhibitory action against mutagens such as sodium azide and 4-nitro-O-phenylene diamine and exhibited an antimutagenic effect [196].
Nonetheless, an investigation documented significant hepatic and renal lesions and central vein abnormalities in rats treated with T. chebula extract [197]. T. chebula fruits (hydroalcoholic extract), through an in vitro study, displayed potential inhibitory action on cytochrome P450 enzymes present in the liver microsome of rats [198].
Lately, a study has been performed to evaluate the safety and examine the toxic effects of T. chebula in which rats were treated with water extract from dried fruits. The acute toxicity study exhibited no visible toxic reactions, for example-mortality, behavioural and histopathological changes in internal organs or changes in gross appearances in rats. The chronic toxicity results did not display any abnormalities in the test groups compared to the controls. In addition, haematological and blood chemical values in test groups were average compared to the control groups [199]. Another study analysed the mutagenicity and oral toxicity in rats demonstrating that the ethyl acetate soluble portion of T. chebula ethanol extract did not produce any adverse effects [192].
10. Limitations of the review
This review focuses on all available activities in the mentioned electronic databases in the methodology section. All available articles may not be included in this review, because of not being indexed in the selected databases. Despite the retrieval of articles included major indexing electronic databases, still some articles may miss out as they not been encountered. Moreover, T. chebula is a commonly used medicinal plant of immense pharmacological importance, therefore the number of available articles are increasing. As a result, some articles may be excluded as they were published after the selected time period of our study.
11. Conclusion
The global appeal for plant-derived medicines is increasing along with the expansion of corresponding laboratory-based analysis. Rapid entrances of numerous drugs of plant origin with a broad spectrum of pharmacological activities into the local and international market have been increased by exploring ethnopharmacology and traditional medicine. Though the demand is high, careful identification and validation of novel agents with their cellular targets, evaluating the efficacy, ensuring safety and toxicity, confirming metabolism and bioavailability with robust clinical studies are some must needed issues to be addressed (Figure 5). With legitimate scientific findings, T. chebula can be remarked as a resourceful plant having a surfeit of therapeutic activities. The broad spectrum of biological activities of T. chebula has been documented in numerous pathophysiological conditions and found to be relatively safe. Several phytochemical studies revealed many chemically diversified biologically active compounds, which need further studies to confirm their therapeutic effects in vitro and in vivo. This review will help the upcoming researchers to develop and implement new ideas on T. chebula. Moreover, future investigations are required to enhance insight into the mechanistic pathways, safety and toxicity, metabolism and bioavailability of the plant, and its bioactive compounds before clinical applications.
Figure 5.
A tentative guidelines for drug development from T. chebula: A laboratory to patient journey.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We acknowledge Afsana Hassan for her support and motivation to conduct this review.
References
- 1.Boadu A.A., Asase A. Documentation of herbal medicines used for the treatment and management of human diseases by some communities in southern Ghana. Evid. base Compl. Alternative Med. 2017;2017 doi: 10.1155/2017/3043061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey A.L. Natural products in drug discovery. Drug Discov. Today. 2008;13(19-20):894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Chopra A. Ayurvedic medicine and arthritis. Rheum. Dis. Clin. N. Am. 2000;26(1):133–144. doi: 10.1016/s0889-857x(05)70127-7. [DOI] [PubMed] [Google Scholar]
- 4.Muthu C., Ayyanar M., Raja N., Ignacimuthu S. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J. Ethnobiol. Ethnomed. 2006;2(1):1–10. doi: 10.1186/1746-4269-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahesh R., Bhuvana S., Hazeena Begum V.M. Effect of Terminalia chebula aqueous extract on oxidative stress and antioxidant status in the liver and kidney of young and aged rats. Cell Biochem. Funct.: Cell. Biochem. Modul. Active Agents Dis. 2009;27(6):358–363. doi: 10.1002/cbf.1581. [DOI] [PubMed] [Google Scholar]
- 6.Datta S., Pal N.K., Nandy A.K. In vitro antibacterial activity of bioactive potent compounds from Terminalia chebula against some common human pathogens. Pharmacol. Pharm. 2017;8(9):283–291. [Google Scholar]
- 7.Bajpai V.K., Rahman A., Shukla S., Shukla S., Yassir Arafat S., Hossain M.A., Mehta A. In vitro kinetics and antifungal activity of various extracts of Terminalia chebula seeds against plant pathogenic fungi. Arch. Phytopathol. Plant Protect. 2010;43(8):801–809. [Google Scholar]
- 8.Messeha S., Zarmouh N., Taka E., Gendy S., Shokry G., Kolta M., Soliman K. The role of monocarboxylate transporters and their chaperone cd147 in lactate efflux inhibition and the anticancer effects of Terminalia chebula in neuroblastoma cell line n2-a. Eur. J. Med. Plants. 2016;12(4) doi: 10.9734/EJMP/2016/23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasidharan I., Sundaresan A., Nisha V.M., Kirishna M.S., Raghu K.G., Jayamurthy P. Inhibitory effect of Terminalia chebula Retz. fruit extracts on digestive enzyme related to diabetes and oxidative stress. J. Enzym. Inhib. Med. Chem. 2012;27(4):578–586. doi: 10.3109/14756366.2011.603130. [DOI] [PubMed] [Google Scholar]
- 10.Sabu M., Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J. Ethnopharmacol. 2002;81(2):155–160. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 11.Manosroi A., Jantrawut P., Akihisa T., Manosroi W., Manosroi J. In vitro anti-aging activities of Terminalia chebula gall extract. Pharmaceut. Biol. 2010;48(4):469–481. doi: 10.3109/13880200903586286. [DOI] [PubMed] [Google Scholar]
- 12.Ahn M.J., Kim C.Y., Lee J.S., Kim T.G., Kim S.H., Lee C.K., Lee B.B., Shin C.G., Huh H., Kim J. Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 2002;68(5):457–459. doi: 10.1055/s-2002-32070. [DOI] [PubMed] [Google Scholar]
- 13.Kumar K. Effect of geographical variation on contents of tannic acid, gallic acid, chebulinic acid and ethyl gallate in Terminalia chebula. Natural Prod. 2006;2(3-4):170–175. [Google Scholar]
- 14.Abir M.H., Rahman T., Das A., Etu S.N., Nafiz I.H., Rakib A., Mitra S., Emran T.B., Dhama K., Islam A. Pathogenicity and virulence of Marburg virus. Virulence. 2022;13(1):609–633. doi: 10.1080/21505594.2022.2054760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusirisin W., Srichairatanakool S., Lerttrakarnnon P., Lailerd N., Suttajit M., Jaikang C., Chaiyasut C. Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med. Chem. 2009;5(2):139–147. doi: 10.2174/157340609787582918. [DOI] [PubMed] [Google Scholar]
- 16.Kannan P., Ramadevi S., Hopper W. Antibacterial activity of Terminalia chebula fruit extract. Afr. J. Microbiol. Res. 2009;3(4):180–184. [Google Scholar]
- 17.Saleem A., Ahotupa M., Pihlaja K. Total phenolics concentration and antioxidant potential of extracts of medicinal plants of Pakistan. Z. Naturforsch. C Biosci. 2001;56(11-12):973–978. doi: 10.1515/znc-2001-11-1211. [DOI] [PubMed] [Google Scholar]
- 18.Li K., Diao Y., Zhang H., Wang S., Zhang Z., Yu B., Huang S., Yang H. Tannin extracts from immature fruits of Terminalia chebula Fructus Retz. promote cutaneous wound healing in rats. BMC Compl. Alternative Med. 2011;11(1):1–9. doi: 10.1186/1472-6882-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleem A., Husheem M., Härkönen P., Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J. Ethnopharmacol. 2002;81(3):327–336. doi: 10.1016/s0378-8741(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A., Mishra A., Bansal P., Singh R., Kumar S., Gupta V. Phytochemistry and pharmacological activities of haritaki-A review. J. Pharm. Res. 2010;3(2):417–424. [Google Scholar]
- 21.Upadhyay A., Agrahari P., Singh D. A review on the pharmacological aspects of Terminalia chebula. Int. J. Pharmacol. 2014;10(6):289–298. [Google Scholar]
- 22.Rathinamoorthy R., Thilagavathi G. Terminalia chebula-review on pharmacological and biochemical studies. Int. J. PharmTech Res. 2014;6(1):97–116. [Google Scholar]
- 23.Jagtap A., Karkera S. Potential of the aqueous extract of Terminalia chebula as an anticaries agent. J. Ethnopharmacol. 1999;68(1-3):299–306. doi: 10.1016/s0378-8741(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 24.Bhatt I.D., Rawat S., Badhani A., Rawal R.S. Nutraceutical potential of selected wild edible fruits of the Indian Himalayan region. Food Chem. 2017;215:84–91. doi: 10.1016/j.foodchem.2016.07.143. [DOI] [PubMed] [Google Scholar]
- 25.Nutalapati C., Chiranjeevi U., Kishan P., Kiran K., Pingali U. A randomized, double-blind, placebo-controlled, parallel group clinical study to evaluate the analgesic effect of aqueous extract of terminalia chebula, a proprietary chromium complex, and their combination in subjects with joint discomfort. Asian J. Pharmaceut. Clin. Res. 2016;9(3):264–269. [Google Scholar]
- 26.Sheng Z., Zhao J., Muhammad I., Zhang Y. Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0202368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh G., Kumar P. Extraction, gas chromatography–mass spectrometry analysis and screening of fruits of Terminalia chebula Retz. for its antimicrobial potential. Pharmacogn. Res. 2013;5(3):162. doi: 10.4103/0974-8490.112421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H., Cho J.-H., Jeong E., Lim J., Lee S., Lee H. Growth-inhibiting activity of active component isolated from Terminalia chebula fruits against intestinal bacteria. J. Food Protect. 2006;69(9):2205–2209. doi: 10.4315/0362-028x-69.9.2205. [DOI] [PubMed] [Google Scholar]
- 29.Naik D.G., Puntambekar H., Anantpure P. Essential oil of Terminalia chebula fruits as a repellent for the Indian honeybee Apis florea. Chem. Biodivers. 2010;7(5):1303–1310. doi: 10.1002/cbdv.200900274. [DOI] [PubMed] [Google Scholar]
- 30.Manosroi A., Jantrawut P., Ogihara E., Yamamoto A., Fukatsu M., Yasukawa K., Tokuda H., Suzuki N., Manosroi J., Akihisa T. Biological activities of phenolic compounds and triterpenoids from the galls of Terminalia chebula. Chem. Biodivers. 2013;10(8):1448–1463. doi: 10.1002/cbdv.201300149. [DOI] [PubMed] [Google Scholar]
- 31.Thanigaivel A., Vasantha-Srinivasan P., Senthil-Nathan S., Edwin E.-S., Ponsankar A., Chellappandian M., Selin-Rani S., Lija-Escaline J., Kalaivani K. Impact of Terminalia chebula Retz. against Aedes aegypti L. and non-target aquatic predatory insects. Ecotoxicol. Environ. Saf. 2017;137:210–217. doi: 10.1016/j.ecoenv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Subramaniyan S., Chennam S., Devi S. Antioxidant activity of ethanolic extract of Terminalia chebula fruit against isoproterenol-induced oxidative stress in rats. Indian J. Biochem. Biophys. 2005;42:246–249. [PubMed] [Google Scholar]
- 33.Chang C.L., Lin C.S. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid. base Compl. Alternative Med.: eCAM. 2012;2012:125247. doi: 10.1155/2012/125247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik G., Priyadarsini K., Naik D., Gangabhagirathi R., Mohan H. Studies on the aqueous extract of Terminalia chebula as a potent antioxidant and a probable radioprotector. Phytomedicine. 2004;11(6):530–538. doi: 10.1016/j.phymed.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Chen X., Sun F., Ma L., Wang J., Qin H., Du G. In vitro evaluation on the antioxidant capacity of triethylchebulate, an aglycone from Terminalia chebula Retz fruit. Indian J. Pharmacol. 2011;43(3):320. doi: 10.4103/0253-7613.81508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedina A., Kotti P., Kausar J., Anand V. Phytopharmacological overview of Terminalia chebula Retz. Phcog. J. 2016;8(4) [Google Scholar]
- 37.Srivastava P., Raut H.N., Wagh R.S., Puntambekar H.M., Kulkarni M.J. Purification and characterization of an antioxidant protein (∼ 16 kDa) from Terminalia chebula fruit. Food Chem. 2012;131(1):141–148. [Google Scholar]
- 38.Birur R.S., Ramachandra Y., Subaramaihha S.R., Subbaiah S.G.P., Austin R.S., Dhananjaya B.L. Antioxidant activities of leaf galls extracts of Terminalia chebula (Gaertn.) Retz.(Combretaceae) Acta Scientiarum Polonorum Technologia Alimentaria. 2015;14(2) doi: 10.17306/J.AFS.2015.2.11. [DOI] [PubMed] [Google Scholar]
- 39.Pfundstein B., El Desouky S.K., Hull W.E., Haubner R., Erben G., Owen R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71(10):1132–1148. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Venkatesan A., Kathirvel A., Prakash S., Sujatha V. Antioxidant, antibacterial activities and identification of bioactive compounds from Terminalia chebula bark extracts. Free Radic. Antioxidants. 2017;7(1):43–49. [Google Scholar]
- 41.Pratibha N., Saxena V., Amit A., D'souza P., Bagchi M., Bagchi D. Anti-inflammatory activities of Aller-7, a novel polyherbal formulation for allergic rhinitis. Int. J. Tissue React. 2004;26(1-2):43–51. [PubMed] [Google Scholar]
- 42.D'Souza P., Amit A., Saxena V., Bagchi D., Bagchi M., Stohs S. Antioxidant properties of Aller-7, a novel polyherbal formulation for allergic rhinitis. Drugs Under Exp. Clin. Res. 2004;30(3):99–109. [PubMed] [Google Scholar]
- 43.Sadeghnia H.R., Jamshidi R., Afshari A.R., Mollazadeh H., Forouzanfar F., Rakhshandeh H. Terminalia chebula attenuates quinolinate-induced oxidative PC12 and OLN-93 cell death. Multi. Scler. Relat. Disorder. 2017;14:60–67. doi: 10.1016/j.msard.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Walia H., Kumar S., Arora S. Comparative analysis of antioxidant and phenolic content of chloroform extract/fraction of Terminalia chebula. J. Basic Clin. Pharm. 2011;2(2):115. [PMC free article] [PubMed] [Google Scholar]
- 45.Tasduq S., Singh K., Satti N., Gupta D., Suri K., Johri R. Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum. Exp. Toxicol. 2006;25(3):111–118. doi: 10.1191/0960327106ht601oa. [DOI] [PubMed] [Google Scholar]
- 46.Sarkar R., Hazra B., Mandal N. Reducing power and iron chelating property of Terminalia chebula (Retz.) alleviates iron induced liver toxicity in mice. BMC Compl. Alternative Med. 2012;12(1):1–10. doi: 10.1186/1472-6882-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramkissoon J., Mahomoodally M., Ahmed N., Subratty A. Antioxidant and anti–glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 2013;6(7):561–569. doi: 10.1016/S1995-7645(13)60097-8. [DOI] [PubMed] [Google Scholar]
- 48.Jami M.S.I., Sultana Z., Ali M.E., Begum M.M., Haque M.M. 2014. Evaluation of Analgesic and Anti-inflammatory Activities on Ethanolic Extract of Terminalia Chebula Fruits in Experimental Animal Models. [Google Scholar]
- 49.Liu W., Mu F., Liu T., Xu H., Chen J., Jia N., Zhang Y., Dou F., Shi L., Li Y., Wen A., Ding Y. Ultra performance liquid chromatography/quadrupole time-of-flight mass spectrometry-based metabonomics reveal protective effect of Terminalia chebula extract on ischemic stroke rats. Rejuvenation Res. 2018;21(6):541–552. doi: 10.1089/rej.2018.2082. [DOI] [PubMed] [Google Scholar]
- 50.Moeslinger T., Friedl R., Volf I., Brunner M., Koller E., Spieckermann P.G. Inhibition of inducible nitric oxide synthesis by the herbal preparation Padma 28 in macrophage cell line. Can. J. Physiol. Pharmacol. 2000;78(11):861–866. [PubMed] [Google Scholar]
- 51.Nair V., Singh S., Gupta Y.K. Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J. Pharm. Pharmacol. 2010;62(12):1801–1806. doi: 10.1111/j.2042-7158.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- 52.Seo J.B., Jeong J.-Y., Park J.Y., Jun E.M., Lee S.-I., Choe S.S., Park D.-Y., Choi E.-W., Seen D.-S., Lim J.-S. Anti-arthritic and analgesic effect of NDI10218, a standardized extract of Terminalia chebula, on arthritis and pain model. Biomol. Therap. 2012;20(1):104. doi: 10.4062/biomolther.2012.20.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murdock N., Gupta C., Vega N., Kotora K., Miller J., Goad T., Lasher A., Canerdy D., Kalidindi S. Evaluation of Terminalia chebula extract for anti-arthritic efficacy and safety in osteoarthritic dogs. J. Vet. Sci. Technol. 2016;7(1) [Google Scholar]
- 54.Sanmuga Priya E., Senthamil Selvan P., Ajay B. Tannin rich fraction from Terminalia chebula fruits as Anti-inflammatory agent. J. Herbs, Spices, Med. Plants. 2018;24(1):74–86. [Google Scholar]
- 55.Bag A., Kumar Bhattacharyya S., Kumar Pal N., Ranjan Chattopadhyay R. Anti-inflammatory, anti-lipid peroxidative, antioxidant and membrane stabilizing activities of hydroalcoholic extract of Terminalia chebula fruits. Pharmaceut. Biol. 2013;51(12):1515–1520. doi: 10.3109/13880209.2013.799709. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y., Liu F., Liu Y., Zhou D., Dai Q., Liu S. Anti-arthritic effect of chebulanin on collagen-induced arthritis in mice. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0139052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nair V., Kumar R., Singh S., Gupta Y. Anti-granuloma activity of Terminalia chebula retz. In wistar rats. Eur. J. Inflamm. 2012;10(2):185–192. [Google Scholar]
- 58.Choudhary M., Kumar V., Malhotra H., Singh S. Medicinal plants with potential anti-arthritic activity. J. Intercul. Ethnopharmacol. 2015;4(2):147. doi: 10.5455/jice.20150313021918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez H., Habowski S., Sandrock J., Raub B., Kedia A., Bruno E., Ziegenfuss T. Effects of dietary supplementation with a standardized aqueous extract of Terminalia chebula fruit (AyuFlex®) on joint mobility, comfort, and functional capacity in healthy overweight subjects: a randomized placebo-controlled clinical trial. BMC Compl. Alternative Med. 2017;17(1):1–18. doi: 10.1186/s12906-017-1977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar G.P.S., Arulselvan P., Kumar D.S., Subramanian S.P. Anti-diabetic activity of fruits of Terminalia chebula on streptozotocin induced diabetic rats. J. Health Sci. 2006;52(3):283–291. [Google Scholar]
- 61.Rao N.K., Nammi S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. seeds in streptozotocin-induced diabetic rats. BMC Compl. Alternative Med. 2006;6(1):1–6. doi: 10.1186/1472-6882-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borgohain R., Lahon K., Das S., Gohain K. Evaluation of mechanism of anti-diabetic activity of Terminalia chebula on alloxan and adrenaline-induced diabetic albino rats. Drugs. 2002;19(5) [Google Scholar]
- 63.Murali Y., Anand P., Tandon V., Singh R., Chandra R., Murthy P. Long-term effects of Terminalia chebula Retz. on hyperglycemia and associated hyperlipidemia, tissue glycogen content and in vitro release of insulin in streptozotocin induced diabetic rats. Exp. Clin. Endocrinol. Diabetes. 2007;115(10):641–646. doi: 10.1055/s-2007-982500. [DOI] [PubMed] [Google Scholar]
- 64.Jing Z., Zeng W., Luo J., Ye H., Huang Y., Gao H. In vitro and in vivo inhibitory effect of methanol extract from Terminalia chebula Retz. fruits on α-glucosidase (In Chinsese) Food Sci. (N. Y.) 2010;31:284–287. [Google Scholar]
- 65.Gao H., Huang Y.-N., Gao B., Kawabata J. Chebulagic acid is a potent α-glucosidase inhibitor. Biosci., Biotechnol., Biochem. 2008;72(2):601–603. doi: 10.1271/bbb.70591. [DOI] [PubMed] [Google Scholar]
- 66.Huang Y.-N., Zhao D.-D., Gao B., Zhong K., Zhu R.-X., Zhang Y., Xie W.-J., Jia L.-R., Gao H. Anti-hyperglycemic effect of chebulagic acid from the fruits of Terminalia chebula Retz. Int. J. Mol. Sci. 2012;13(5):6320–6333. doi: 10.3390/ijms13056320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao H., Huang Y.-N., Xu P.-Y., Kawabata J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 2007;105(2):628–634. [Google Scholar]
- 68.Silawat N., Gupta V.B. Chebulic acid attenuates ischemia reperfusion induced biochemical alteration in diabetic rats. Pharmaceut. Biol. 2013;51(1):23–29. doi: 10.3109/13880209.2012.698288. [DOI] [PubMed] [Google Scholar]
- 69.Kim J.-h., Hong C.-O., Koo Y.-c., Kim S.-J., Lee K.-W. Oral administration of ethyl acetate-soluble portion of Terminalia chebula conferring protection from streptozotocin-induced diabetic mellitus and its complications. Biol. Pharm. Bull. 2011;34(11):1702–1709. doi: 10.1248/bpb.34.1702. [DOI] [PubMed] [Google Scholar]
- 70.Balakrishna V., Lakshmi T. Hepatoprotective activity of ethanolic extract of Terminalia chebula fruit against ethanol-induced hepatotoxicity in rats. Asian J. Pharmaceut. Clin. Res. 2017;10(11):55–58. [Google Scholar]
- 71.Choi M.-K., Kim H.-G., Han J.-M., Lee J.-S., Lee J.S., Chung S.H., Son C.-G. Hepatoprotective effect of Terminalia chebula against t-BHP-induced acute liver injury in C57/BL6 mice. Evid. base Compl. Alternative Med. 2015;2015 doi: 10.1155/2015/517350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olennikov D.N., Kashchenko N.I., Chirikova N.K. In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: a case of Padma Hepaten® formulation. Nutrients. 2015;7(10):8456–8477. doi: 10.3390/nu7105406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li N., Li B., Zhang J., Liu X., Liu J., Li K., Pan T., Wang S., Diao Y. Protective effect of phenolic acids from Chebulae Fructus immaturus on carbon tetrachloride induced acute liver injury via suppressing oxidative stress, inflammation and apoptosis in mouse. Nat. Prod. Res. 2020;34(22):3249–3252. doi: 10.1080/14786419.2018.1553174. [DOI] [PubMed] [Google Scholar]
- 74.Matkowski A., Jamiolkowska-Kozlowska W., Nawrot I. Chinese medicinal herbs as source of antioxidant compounds–where tradition meets the future. Curr. Med. Chem. 2013;20(8):984–1004. [PubMed] [Google Scholar]
- 75.Leelawat S., Leelawat K. Molecular mechanisms of cholangiocarcinoma cell inhibition by medicinal plants. Oncol. Lett. 2017;13(2):961–966. doi: 10.3892/ol.2016.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shankara B.R., Ramachandra Y., Rajan S.S., Ganapathy P.S., Yarla N.S., Richard S., Dhananjaya B.L. Evaluating the anticancer potential of ethanolic gall extract of Terminalia chebula (Gaertn.) Retz.(combretaceae) Pharmacogn. Res. 2016;8(3):209. doi: 10.4103/0974-8490.182919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reddy D.B., Reddy T., Jyotsna G., Sharan S., Priya N., Lakshmipathi V., Reddanna P. Chebulagic acid, a COX–LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J. Ethnopharmacol. 2009;124(3):506–512. doi: 10.1016/j.jep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 78.Pellati F., Bruni R., Righi D., Grandini A., Tognolini M., Prencipe F.P., Poli F., Benvenuti S., Del Rio D., Rossi D. Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J. Ethnopharmacol. 2013;147(2):277–285. doi: 10.1016/j.jep.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 79.Yoshioka K., Kataoka T., Hayashi T., Hasegawa M., Ishi Y., Hibasami H. Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO 205 cell lines. Oncol. Rep. 2000;7(6):1221–1224. doi: 10.3892/or.7.6.1221. [DOI] [PubMed] [Google Scholar]
- 80.Lee S.-H., Ryu S.Y., Choi S.U., Lee C.O., No Z., Kim S.-K., Ahn J.-W. Hydrolysable tannins and related compound having cytotoxic activity from the fruits of Terminalia chebula. Arch Pharm. Res. (Seoul) 1995;18(2):118–120. [Google Scholar]
- 81.Wani K., Shah N., Prabhune A., Jadhav A., Ranjekar P., Kaul-Ghanekar R. Evaluating the anticancer activity and nanoparticulate nature of homeopathic preparations of Terminalia chebula. Homeopathy. 2016;105(4):318–326. doi: 10.1016/j.homp.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Ravinayagam V., Jaganathan R., Panchanadham S., Palanivelu S. Cytotoxic effect of tridham (TD) against human HepG2 Cell line: isolation and characterization of 3, 4, 5-trihydroxybenzoic acid from aqueous extract of TD. IJDDHR. 2012;2(2):371–385. [Google Scholar]
- 83.Shendge A.K., Sarkar R., Mandal N. Potent anti-inflammatory Terminalia chebula fruit showed in vitro anticancer activity on lung and breast carcinoma cells through the regulation of Bax/Bcl-2 and caspase-cascade pathways. J. Food Biochem. 2020;44(12) doi: 10.1111/jfbc.13521. [DOI] [PubMed] [Google Scholar]
- 84.Kumar N., Gangappa D., Gupta G., Karnati R. Chebulagic acid from Terminalia chebula causes G1 arrest, inhibits NFκB and induces apoptosis in retinoblastoma cells. BMC Compl. Alternative Med. 2014;14(1):1–10. doi: 10.1186/1472-6882-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reddy M.M., Dhas Devavaram J., Dhas J., Adeghate E., Starling Emerald B. Anti-hyperlipidemic effect of methanol bark extract of Terminalia chebula in male albino Wistar rats. Pharmaceut. Biol. 2015;53(8):1133–1140. doi: 10.3109/13880209.2014.962058. [DOI] [PubMed] [Google Scholar]
- 86.Chhabra S., Mishra T., Kumar Y., Thacker G., Kanojiya S., Chattopadhyay N., Narender T., Trivedi A.K. Chebulinic acid isolated from the fruits of Terminalia chebula specifically induces apoptosis in acute myeloid leukemia cells. Phytother Res. 2017;31(12):1849–1857. doi: 10.1002/ptr.5927. [DOI] [PubMed] [Google Scholar]
- 87.Bupesh G., Manikandan E., Thanigaiarul K., Magesh S., Senthilkumar V. Enhanced antibacterial, anticancer activity from Terminalia chebula. Medicinal plant rapid extract by phytosynthesis of silver nanoparticles core-shell structures. J. Nanomed. Nanotechnol. 2016;7:355. [Google Scholar]
- 88.Achari C., V Reddy G., CM Reddy T., Reddanna P. Chebulagic acid synergizes the cytotoxicity of doxorubicin in human hepatocellular carcinoma through COX-2 dependant modulation of MDR-1. Med. Chem. 2011;7(5):432–442. doi: 10.2174/157340611796799087. [DOI] [PubMed] [Google Scholar]
- 89.Shaila H., Udupa S., Udupa A. Hypolipidemic activity of three indigenous drugs in experimentally induced atherosclerosis. Int. J. Cardiol. 1998;67(2):119–124. doi: 10.1016/s0167-5273(98)00281-2. [DOI] [PubMed] [Google Scholar]
- 90.Rathore H., Soni S., Bhatnagar D. Hypocholesterolemic effect of Terminalia chebula fruit (Myrobalan) in mice. Ancient Sci. Life. 2004;23(4):11. [PMC free article] [PubMed] [Google Scholar]
- 91.Israni D.A., Patel K.V., Gandhi T.R. Anti-hyperlipidemic activity of aqueous extract of Terminalia chebula & Gaumutra in high cholesterol diet fed rats. Pharma Sci. Monit. 2010;1(1):48–59. [Google Scholar]
- 92.Suchalatha S., Devi C. 2004. Protective Effect of Terminalia Chebula against Experimental Myocardial Injury Induced by Isoproterenol. [PubMed] [Google Scholar]
- 93.Suchalatha S., Devi C.S.S. Protective effect of Terminalia chebula against lysosomal enzyme alterations in isoproterenol-induced cardiac damage in rats. Exp. Clin. Cardiol. 2005;10(2):91. [PMC free article] [PubMed] [Google Scholar]
- 94.Suchalatha S., Srinivasan P., Devi C.S.S. Effect of T. chebula on mitochondrial alterations in experimental myocardial injury. Chem. Biol. Interact. 2007;169(3):145–153. doi: 10.1016/j.cbi.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 95.Reddy V.R.C., Kumari S.R., Reddy B.M., Azeem M., Prabhakar M., Rao A.A. Cardiotonic activity of the fruits of Terminalia chebula. Fitoterapia. 1990;41(6):517–525. [Google Scholar]
- 96.Sharma P., Prakash T., Kotresha D., Ansari M.A., Sahrm U.R., Kumar B., Debnath J., Goli D. Antiulcerogenic activity of Terminalia chebula fruit in experimentally induced ulcer in rats. Pharmaceut. Biol. 2011;49(3):262–268. doi: 10.3109/13880209.2010.503709. [DOI] [PubMed] [Google Scholar]
- 97.Mishra V., Agrawal M., Onasanwo S.A., Madhur G., Rastogi P., Pandey H.P., Palit G., Narender T. Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers. Phytomedicine. 2013;20(6):506–511. doi: 10.1016/j.phymed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Suguna L., Singh S., Sivakumar P., Sampath P., Chandrakasan G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother Res. 2002;16(3):227–231. doi: 10.1002/ptr.827. [DOI] [PubMed] [Google Scholar]
- 99.Raju D., Ilango K., Chitra V., Ashish K. Evaluation of Anti-ulcer activity of methanolic extract of Terminalia chebula fruits in experimental rats. J. Pharmaceut. Sci. Res. 2009;1(3):101. [Google Scholar]
- 100.Jokar A., Masoomi F., Sadeghpour O., Nassiri-Toosi M., Hamedi S. Potential therapeutic applications for Terminalia chebula in Iranian traditional medicine. J. Tradit. Chin. Med. 2016;36(2):250–254. doi: 10.1016/s0254-6272(16)30035-8. [DOI] [PubMed] [Google Scholar]
- 101.Liu W., Shang P., Liu T., Xu H., Ren D., Zhou W., Wen A., Ding Y. Gastroprotective effects of chebulagic acid against ethanol-induced gastric injury in rats. Chem. Biol. Interact. 2017;278:1–8. doi: 10.1016/j.cbi.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 102.Safavi M., Shams-Ardakani M.R., Seyedbagheri M.S., Foroumadi A. The efficacy of Iranian traditional and folk medicinal plants for some gastroduodenal disorders. Trad. Integrat. Med. 2016:3–17. [Google Scholar]
- 103.Zaidi S.F., Muhammad J.S., Shahryar S., Usmanghani K., Gilani A.-H., Jafri W., Sugiyama T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol. 2012;141(1):403–410. doi: 10.1016/j.jep.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 104.Nariya M., Shukla V., Jain S., Ravishankar B. Comparison of enteroprotective efficacy of triphala formulations (Indian Herbal Drug) on methotrexate-induced small intestinal damage in rats. Phytother Res.: Int. J. Dev. Pharmacol. Toxicol. Eval. Natural Prod. Deriv. 2009;23(8):1092–1098. doi: 10.1002/ptr.2744. [DOI] [PubMed] [Google Scholar]
- 105.Izzo A., Carlo G.D., Mascolo N., Capasso F., Autore G. Antiulcer effect of flavonoids. Role of endogenous PAF. Phytother Res. 1994;8(3):179–181. [Google Scholar]
- 106.Parmar N., Parmar S. Anti-ulcer potential of flavonoids. Indian J. Physiol. Pharmacol. 1998;42:343–351. [PubMed] [Google Scholar]
- 107.Sheela V.S., Selvaumar S., Shanthi G. Antiulcer activity of Cayratia pedata, Enicostemma axillare and Terminalia chebula on ethanol induced Albino Wister rats. Int. J. Res. Rev. 2016;4:1129–1134. [Google Scholar]
- 108.Shen Y.-C., Juan C.-W., Lin C.-S., Chen C.-C., Chang C.-L. Neuroprotective effect of Terminalia chebula extracts and ellagic acid in pc12 cells. Afr. J. Tradit., Complementary Altern. Med. 2017;14(4):22–30. doi: 10.21010/ajtcam.v14i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sancheti S., Um B.-H., Seo S.-Y. 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose: a cholinesterase inhibitor from Terminalia chebula. South Afr. J. Bot. 2010;76(2):285–288. [Google Scholar]
- 110.Nag G., De B. Acetylcholinesterase inhibitory activity of Terminalia chebula, Terminalia bellerica and Emblica officinalis and some phenolic compounds. Int. J. Pharm. Pharmaceut. Sci. 2011;3(3):121–124. [Google Scholar]
- 111.Afshari A.R., Sadeghnia H.R., Mollazadeh H. A review on potential mechanisms of Terminalia chebula in Alzheimer’s disease. Adv. Pharmacol. Sci. 2016;2016 doi: 10.1155/2016/8964849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murray A.P., Faraoni M.B., Castro M.J., Alza N.P., Cavallaro V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropzarmacol. 2013;11(4):388–413. doi: 10.2174/1570159X11311040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pugazhendhi A., Shafreen R.B., Devi K.P., Suganthy N. Assessment of antioxidant, anticholinesterase and antiamyloidogenic effect of Terminalia chebula, Terminalia arjuna and its bioactive constituent 7-methyl gallic acid–an in vitro and in silico studies. J. Mol. Liq. 2018;257:69–81. [Google Scholar]
- 114.Park J.H., Joo H.S., Yoo K.-Y., Shin B.N., Kim I.H., Lee C.H., Choi J.H., Byun K., Lee B., Lim S.S. Extract from Terminalia chebula seeds protect against experimental ischemic neuronal damage via maintaining SODs and BDNF levels. Neurochem. Res. 2011;36(11):2043–2050. doi: 10.1007/s11064-011-0528-9. [DOI] [PubMed] [Google Scholar]
- 115.Kanna S., Hiremath S., Unger B.S. Nephroprotective activity of Bilvādi agada in gentamicin induced nephrotoxicity in male Wistar rats. Ancient Sci. Life. 2015;34(3):126. doi: 10.4103/0257-7941.157146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chandrasekhar Y., Phani Kumar G., Navya K., Ramya E.M., Anilakumar K.R. Tannins from Terminalia chebula fruits attenuates GABA antagonist-induced anxiety-like behaviour via modulation of neurotransmitters. J. Pharm. Pharmacol. 2018;70(12):1662–1674. doi: 10.1111/jphp.13007. [DOI] [PubMed] [Google Scholar]
- 117.Kim H.J., Kim J., Kang K.S., Lee K.T., Yang H.O. Neuroprotective effect of chebulagic acid via autophagy induction in SH-SY5Y cells. Biomol. Therapeut. 2014;22(4):275. doi: 10.4062/biomolther.2014.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Debnath J., Sharma U.R., Kumar B., Chauhan N.S. Anticonvulsant activity of ethanolic extract of fruits of Terminalia chebula on experimental animals. Int. J. Drug Dev. Res. 2010;2(4):764–768. [Google Scholar]
- 119.Kumar R., Arora R., Agarwal A., Gupta Y. Protective effect of Terminalia chebula against seizures, seizure-induced cognitive impairment and oxidative stress in experimental models of seizures in rats. J. Ethnopharmacol. 2018;215:124–131. doi: 10.1016/j.jep.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 120.Nigam M., Mishra A.P., Adhikari-Devkota A., Dirar A.I., Hassan M.M., Adhikari A., Belwal T., Devkota H.P. Fruits of Terminalia chebula Retz.: a review on traditional uses, bioactive chemical constituents and pharmacological activities. Phytother Res. 2020;34(10):2518–2533. doi: 10.1002/ptr.6702. [DOI] [PubMed] [Google Scholar]
- 121.Tayal S., Duggal S., Bandyopadhyay P., Aggarwal A., Tandon S., Tandon C. Cytoprotective role of the aqueous extract of Terminalia chebula on renal epithelial cells. Int. Braz J. Urol. 2012;38:204–214. doi: 10.1590/s1677-55382012000200008. [DOI] [PubMed] [Google Scholar]
- 122.Gopi K., Reddy A.G., Jyothi K., Kumar B.A. Acetaminophen-induced hepato-and nephrotoxicity and amelioration by silymarin and Terminalia chebula in rats. Toxicol. Int. 2010;17(2):64. doi: 10.4103/0971-6580.72672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yadav H.N., Sharma U.S., Singh S., Gupta Y.K. Effect of combination of Tribulus terrestris, Boerhavia diffusa and Terminalia chebula reverses mercuric chloride-induced nephrotoxicity and renal accumulation of mercury in rat. Orien. Pharm. Experim. Med. 2019;19(4):497–507. [Google Scholar]
- 124.Prasad L., Khan T., Jahangir T., Sultana S. Abrogation of DEN/Fe-NTA induced carcinogenic response, oxidative damage and subsequent cell proliferation response by Terminalia chebula in kidney of Wistar rats. Die Pharmazie-An Int. J. Pharmaceut. Sci. 2007;62(10):790–797. [PubMed] [Google Scholar]
- 125.Muneer A., Alhowail A., Aldubayan M., Rabbani S.I. The activity of Terminalia chebula Retz. extract on doxorubicin-induced renal damage in rats. J. Pharm. Pharmacogn. Res. 2020;8(3):237–246. [Google Scholar]
- 126.Upadhyay A., Singh D. Molluscicidal activity of Sapindus mukorossi and Terminalia chebula against the freshwater snail Lymnaea acuminata. Chemosphere. 2011;83(4):468–474. doi: 10.1016/j.chemosphere.2010.12.066. [DOI] [PubMed] [Google Scholar]
- 127.Gupta P.C. Biological and pharmacological properties of Terminalia chebula Retz.(Haritaki)-An overview. Int. J. Pharm. Pharmaceut. Sci. 2012;4(3):62–68. [Google Scholar]
- 128.Singh D., Agarwal R. In vivo and in vitro studies on synergism with anticholinesterase pesticides in the snail Lymnaea acuminata. Arch. Environ. Contam. Toxicol. 1983;12(4):483–487. [Google Scholar]
- 129.Upadhyay A., Singh D.K. Inhibition kinetics of certain enzymes in the nervous tissue of vector snail Lymnaea acuminata by active molluscicidal components of Sapindus mukorossi and Terminalia chebula. Chemosphere. 2011;85(6):1095–1100. doi: 10.1016/j.chemosphere.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 130.Singh A., Singh D., Misra T., Agarwal R. Molluscicides of plant origin. Biol. Agric. Hortic. 1996;13,(3):205–252. [Google Scholar]
- 131.Marston A., Hostettmann K. Review article number 6: plant molluscicides. Phytochemistry. 1985;24(4):639–652. [Google Scholar]
- 132.Dixit D., Dixit A.K., Lad H., Gupta D., Bhatnagar D. Radioprotective effect of Terminalia Chebula Retzius extract against γ-irradiation-induced oxidative stress. Biomed. Aging Pathol. 2013;3(2):83–88. [Google Scholar]
- 133.Gandhi N.M., Nair C.K.K. Radiation protection by Terminalia chebula: some mechanistic aspects. Mol. Cell. Biochem. 2005;277(1):43–48. doi: 10.1007/s11010-005-4819-9. [DOI] [PubMed] [Google Scholar]
- 134.Nasiri E., Hosseinimehr S.J., Azadbakht M., Akbari J., Enayati-Fard R., Azizi S. The effect of Terminalia chebula extract vs. silver sulfadiazine on burn wounds in rats. J. Compl. Integr. Med. 2015;12(2):127–135. doi: 10.1515/jcim-2014-0068. [DOI] [PubMed] [Google Scholar]
- 135.Lee H.-H., Paudel K.R., Kim D.-W. Terminalia chebula fructus inhibits migration and proliferation of vascular smooth muscle cells and production of inflammatory mediators in RAW 264.7. Evid. base Compl. Alternative Med. 2015;2015 doi: 10.1155/2015/502182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh M.P., Sharma C.S. Wound healing activity of Terminalia chebula in experimentally induced diabetic rats. Int. J. PharmTech Res. 2009;1(4):1267–1270. [Google Scholar]
- 137.Singh D., Singh D., Choi S.M., Zo S.M., Painuli R.M., Kwon S.W., Han S.S. Effect of extracts of Terminalia chebula on proliferation of keratinocytes and fibroblasts cells: an alternative approach for wound healing. Evid. base Compl. Alternative Med. 2014;2014 doi: 10.1155/2014/701656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singh A., Srivastav R., Pandey A.K. Protective role of Terminalia Chebula in streptozotocin-induced diabetic mice for wound healing activity. J. Adv. Med. Med. Res. 2017:1–8. [Google Scholar]
- 139.Na M., Bae K., Kang S.S., Min B.S., Yoo J.K., Kamiryo Y., Senoo Y., Yokoo S., Miwa N. Cytoprotective effect on oxidative stress and inhibitory effect on cellular aging of Terminalia chebula fruit. Phytother Res.: PTR. 2004;18(9):737–741. doi: 10.1002/ptr.1529. [DOI] [PubMed] [Google Scholar]
- 140.Yakaew S., Itsarasook K., Ngoenkam J., Jessadayannamaetha A., Viyoch J., Ungsurungsie M. Ethanol extract of Terminalia chebula fruit protects against UVB-induced skin damage. Pharmaceut. Biol. 2016;54(11):2701–2707. doi: 10.1080/13880209.2016.1179768. [DOI] [PubMed] [Google Scholar]
- 141.Lee H.S., Koo Y.C., Suh H.J., Kim K.Y., Lee K.W. Preventive effects of chebulic acid isolated from Terminalia chebula on advanced glycation endproduct-induced endothelial cell dysfunction. J. Ethnopharmacol. 2010;131(3):567–574. doi: 10.1016/j.jep.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 142.Hamada S.-i., Kataoka T., Woo J.-T., Yamada A., Yoshida T., Nishimura T., Otake N., Nagai K. Immunosuppressive effects of gallic acid and chebulagic acid on CTL-mediated cytotoxicity. Biol. Pharm. Bull. 1997;20(9):1017–1019. doi: 10.1248/bpb.20.1017. [DOI] [PubMed] [Google Scholar]
- 143.Sevillano L.G., Pellón M., Lobato C.T., Gil M.S., Valdivieso I.S. Knowledge upon the emergency contraceptive pill in Spain. Eur. J. Clin. Pharm.: Atencion Farm. 2014;16(3):224–228. [Google Scholar]
- 144.Bhattacharya S., Chaudhuri S.R., Chattopadhyay S., Bandyopadhyay S.K. Healing properties of some Indian medicinal plants against indomethacin-induced gastric ulceration of rats. J. Clin. Biochem. Nutr. 2007;41(2):106–114. doi: 10.3164/jcbn.2007015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Debnath J., Prakash T., Karki R., Kotresha D., Sharma P. An experimental evaluation of anti-stress effects of Terminalia chebula. J. Physiol. Biomed. Sci. 2011;24(2):13–19. [Google Scholar]
- 146.Gupta P.C. Reversible contraceptive potential of harad in male albino mice. Int. J. Pharm. Pharmaceut. Sci. 2017;9:288–296. [Google Scholar]
- 147.Ghosh A., Pakhira B.P., Tripathy A., Ghosh D. Male contraceptive efficacy of poly herbal formulation, contracept-TM, composed of aqueous extracts of Terminalia chebula fruit and Musa balbisiana seed in rat. Pharmaceut. Biol. 2017;55(1):2035–2042. doi: 10.1080/13880209.2017.1357734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mani V., Sajid S., Rabbani S.I., Alqasir A.S., Alharbi H.A., Alshumaym A. Anxiolytic-like and antidepressant-like effects of ethanol extract of Terminalia chebula in mice. J. Trad. Complemen. Med. 2021 doi: 10.1016/j.jtcme.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Onasanwo S., Faborode S., Agrawal M., Ijiwola O., Jaiyesimi B., Narender T. Antidepressant and anxiolytic potentials of chebulinic acid in laboratory rodent. Ann. Depress. Anxiety. 2014;1(7):1032. [Google Scholar]
- 150.Sheng Z., Yan X., Zhang R., Ni H., Cui Y., Ge J., Shan A. Assessment of the antidiarrhoeal properties of the aqueous extract and its soluble fractions of Chebulae Fructus (Terminalia chebula fruits) Pharmaceut. Biol. 2016;54(9):1847–1856. doi: 10.3109/13880209.2015.1131993. [DOI] [PubMed] [Google Scholar]
- 151.Mussarat S., Adnan M., Begum S., Rehman S.U., Hashem A., Abd_Allah E.F. Antimicrobial screening of polyherbal formulations traditionally used against gastrointestinal diseases. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parekh J., Chanda S. Evaluation of antimicrobial activity of Terminalia chebula Retz. Fruit in different solvents. J. Herbs, Spices, Med. Plants. 2008;13(2):107–116. [Google Scholar]
- 153.Bag A., Chattopadhyay R.R. Efflux-pump inhibitory activity of a gallotannin from Terminalia chebula fruit against multidrug-resistant uropathogenic Escherichia coli. Nat. Prod. Res. 2014;28(16):1280–1283. doi: 10.1080/14786419.2014.895729. [DOI] [PubMed] [Google Scholar]
- 154.Sarabhai S., Harjai K., Sharma P., Capalash N. Ellagic acid derivatives from Terminalia chebula Retz. increase the susceptibility of Pseudomonas aeruginosa to stress by inhibiting polyphosphate kinase. J. Appl. Microbiol. 2015;118(4):817–825. doi: 10.1111/jam.12733. [DOI] [PubMed] [Google Scholar]
- 155.Acharyya S., Sarkar P., Saha D.R., Patra A., Ramamurthy T., Bag P.K. Intracellular and membrane-damaging activities of methyl gallate isolated from Terminalia chebula against multidrug-resistant Shigella spp. J. Med. Microbiol. 2015;64(8):901–909. doi: 10.1099/jmm.0.000107. [DOI] [PubMed] [Google Scholar]
- 156.Badmaev V., Nowakowski M. Protection of epithelial cells against influenza A virus by a plant derived biological response modifier Ledretan-96. Phytother Res.: Int. J. Dev. Pharmacol. Toxicol. Eval. Natural Prod. Deriv. 2000;14(4):245–249. doi: 10.1002/1099-1573(200006)14:4<245::aid-ptr571>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 157.Kurokawa M., Nagasaka K., Hirabayashi T., Uyama S.-I., Sato H., Kageyama T., Kadota S., Ohyama H., Hozumi T., Namba T. Efficacy of traditional herbal medicines in combination with acyclovir against herpes simplex virus type 1 infection in vitro and in vivo. Antivir. Res. 1995;27(1-2):19–37. doi: 10.1016/0166-3542(94)00076-k. [DOI] [PubMed] [Google Scholar]
- 158.Oyuntsetseg N., Khasnatinov M.A., Molor-Erdene P., Oyunbileg J., Liapunov A.V., Danchinova G.A., Oldokh S., Baigalmaa J., Chimedragchaa C. Evaluation of direct antiviral activity of the Deva-5 herb formulation and extracts of five Asian plants against influenza A virus H3N8. BMC Compl. Alternative Med. 2014;14(1):1–9. doi: 10.1186/1472-6882-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yukawa T.A., Kurokawa M., Sato H., Yoshida Y., Kageyama S., Hasegawa T., Namba T., Imakita M., Hozumi T., Shiraki K. Prophylactic treatment of cytomegalovirus infection with traditional herbs. Antivir. Res. 1996;32(2):63–70. doi: 10.1016/0166-3542(95)00978-7. [DOI] [PubMed] [Google Scholar]
- 160.Suthienkul O., Miyazaki O., Chulasiri M., Kositanont U., Oishi K. Retroviral reverse transcriptase inhibitory activity in Thai herbs and spices: screening with Moloney murine leukemia viral enzyme. Southeast Asian J. Trop. Med. Publ. Health. 1993;24(4):751–755. [PubMed] [Google Scholar]
- 161.EL-Mekkawy S., Meselhy M.R., Kusumoto I.T., KADoTA S., Hattori M., Namba T. Inhibitory effects of egyptian folk medicines oh human immunodeficiency virus (HIV) reverse transcriptase. Chem. Pharm. Bull. 1995;43(4):641–648. doi: 10.1248/cpb.43.641. [DOI] [PubMed] [Google Scholar]
- 162.Chattopadhyay R., Bhattacharyya S. Plant review Terminalia chebula. Phcog. Rev. 2007;23:145–150. [Google Scholar]
- 163.Xie F. Broad-spectrum antiviral effect of chebulagic acid and punicalagin on respiratory syncytial virus infection in a BALB/c model. Int. J. Clin. Exp. Pathol. 2016;9(2):611–619. [Google Scholar]
- 164.Yang Y., Xiu J., Liu J., Zhang L., Li X., Xu Y., Qin C., Zhang L. Chebulagic acid, a hydrolyzable tannin, exhibited antiviral activity in vitro and in vivo against human enterovirus 71. Int. J. Mol. Sci. 2013;14(5):9618–9627. doi: 10.3390/ijms14059618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kesharwani A., Polachira S.K., Nair R., Agarwal A., Mishra N.N., Gupta S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Compl. Alternative Med. 2017;17(1):1–11. doi: 10.1186/s12906-017-1620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vermani K., Garg S. Herbal medicines for sexually transmitted diseases and AIDS. J. Ethnopharmacol. 2002;80(1):49–66. doi: 10.1016/s0378-8741(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 167.Kim T.G., Kang S.Y., Jung K.K., Kang J.H., Lee E., Han H.M., Kim S.H. Antiviral activities of extracts isolated from Terminalis chebula Retz., Sanguisorba officinalis L., Rubus coreanus Miq. and Rheum palmatum L. against hepatitis B virus. Phytother Res. 2001;15(8):718–720. doi: 10.1002/ptr.832. [DOI] [PubMed] [Google Scholar]
- 168.Ma H., Diao Y., Zhao D., Li K., Kang T. A new alternative to treat swine influenza A virus infection: extracts from Terminalia chebula Retz. Afr. J. Microbiol. Res. 2010;4(6):497–499. [Google Scholar]
- 169.Lee D., Boo K.-H., Woo J.-K., Duan F., Lee K.-H., Kwon T.K., Lee H.Y., Riu K.Z., Lee D.-S. Anti-bacterial and anti-viral activities of extracts from Terminalia chebula barks. J. Korean Soc. Appl. Biol. Chem. 2011;54(2):295–298. [Google Scholar]
- 170.Vonshak A., Barazani O., Sathiyamoorthy P., Shalev R., Vardy D., Golan-Goldhirsh A. Screening South Indian medicinal plants for antifungal activity against cutaneous pathogens. Phytother Res.: Int. J. Dev. Pharmacol. Toxicol. Eval. Natural Prod. Deriv. 2003;17(9):1123–1125. doi: 10.1002/ptr.1399. [DOI] [PubMed] [Google Scholar]
- 171.Singh G., Kumar P., Joshi S.C. Treatment of dermatophytosis by a new antifungal agent ‘apigenin. Mycoses. 2014;57(8):497–506. doi: 10.1111/myc.12188. [DOI] [PubMed] [Google Scholar]
- 172.Kabir M.L., Ali M.T., Haque M.E. Antimicrobial, antifungal & cytotoxic activities screening of stem bark fractions from Terminalia chebula. Univ. J. Plant Sci. 2017;5(3):41–44. [Google Scholar]
- 173.Kamaraj C., Rahuman A.A. Efficacy of anthelmintic properties of medicinal plant extracts against Haemonchus contortus. Res. Vet. Sci. 2011;91(3):400–404. doi: 10.1016/j.rvsc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 174.Pinmai K., Hiriote W., Soonthornchareonnon N., Jongsakul K., Sireeratawong S., Tor-Udom S. In vitro and in vivo antiplasmodial activity and cytotoxicity of water extracts of Phyllanthus emblica, Terminalia chebula, and Terminalia bellerica. J. Med. Assoc. Thail. 2011;93(12):120. [PubMed] [Google Scholar]
- 175.Kamaraj C., Rahuman A.A., Bagavan A., Elango G., Rajakumar G., Zahir A.A., Marimuthu S., Santhoshkumar T., Jayaseelan C. Evaluation of medicinal plant extracts against blood-sucking parasites. Parasitol. Res. 2010;106(6):1403–1412. doi: 10.1007/s00436-010-1816-z. [DOI] [PubMed] [Google Scholar]
- 176.Nirmal S., Gagare P., Dighe S., Kadam S., Ishatake S. Anthelmintic activity of some existing polyherbal Ayurvedic formulations. Pharmacologyonline. 2008;3:76–79. [Google Scholar]
- 177.Lokesh R., Veerakumari L. EFfect of ethanol extract of terminalia chebula on the motility and acetylcholinesterase of cotylophoron cotylophorum. Int. J. Pharmaceut. Sci. Res. 2015;6(9):3975–3980. [Google Scholar]
- 178.Roy P., Saha S.K., Gayen P., Chowdhury P., Babu S.P.S. Exploration of antifilarial activity of gold nanoparticle against human and bovine filarial parasites: a nanomedicinal mechanistic approach. Colloids Surf. B Biointerfaces. 2018;161:236–243. doi: 10.1016/j.colsurfb.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 179.Tripathi V., Tewari S., Gupta J., Chaturvedi G. Clinical trial of haritaki (Terminalia chebula) in treatment of simple constipation. Sachittar Ayurveda. 1983;35:733–740. [Google Scholar]
- 180.Gupta D., Bhaskar D., Gupta R.K., Karim B., Gupta V., Punia H., Batra M., Jain A., Agarwal A., Singh P. Effect of Terminalia chebula extract and chlorhexidine on salivary pH and periodontal health: 2 weeks randomized control trial. Phytother Res. 2014;28(7):992–998. doi: 10.1002/ptr.5075. [DOI] [PubMed] [Google Scholar]
- 181.Amit A., Saxena V., Pratibha N., Bagchi M., Bagchi D., Stohs S. Safety of a novel botanical extract formula for ameliorating allergic rhinitis. Toxicol. Mech. Methods. 2003;13(4):253–261. doi: 10.1080/713857188. [DOI] [PubMed] [Google Scholar]
- 182.Mukherjee P.K., Rai S., Bhattachar S., Kumar D.P., Biswas T.K., Jana U., Pandit S., Saha B.P., Paul P.K. 2006. Clinical Study of ‘TRIPHALA’–A Well Known Phytomedicine from india. [Google Scholar]
- 183.Singh R., Sinha B. Clinical and physiological studies on the effect of an indigenous compound Rasayana drug in apparently normal aged person. J. Res. Educ. Indian Med. 1978;13:1–8. [Google Scholar]
- 184.Trivedi B. A clinical trial on Mentat. Probe. 1999;38(4):226. [Google Scholar]
- 185.Kumar C.U., Pokuri V.K., Pingali U. Evaluation of the analgesic activity of standardized aqueous extract of Terminalia Chebula in healthy human participants using hot air pain model. J. Clin. Diagn. Res.: J. Clin. Diagn. Res. 2015;9(5):FC01. doi: 10.7860/JCDR/2015/11369.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pokuri V.K., Kumar C.U., Pingali U. A randomized, double-blind, placebo-controlled, cross-over study to evaluate analgesic activity of Terminalia chebula in healthy human volunteers using a mechanical pain model. J. Anaesthesiol. Clin. Pharmacol. 2016;32(3):329. doi: 10.4103/0970-9185.173365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rege N.N., Thatte U.M., Dahanukar S.A. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res.: Int. J. Dev. Pharmacol. Toxicol. Eval. Natural Prod. Deriv. 1999;13(4):275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 188.Suganthy N., Muniasamy S., Archunan G. Safety assessment of methanolic extract of Terminalia chebula fruit, Terminalia arjuna bark and its bioactive constituent 7-methyl gallic acid: in vitro and in vivo studies. Regul. Toxicol. Pharmacol. 2018;92:347–357. doi: 10.1016/j.yrtph.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 189.Ahmad I., Mehmood Z., Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998;62(2):183–193. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 190.Panunto W., Jaijoy K., Lerdvuthisopon N., Lertprasertsuke N., Jiruntanat N., Soonthornchareonnon N., Sireeratawong S. Acute and chronic toxicity studies of the water extract from dried fruits of Terminalia chebula Rezt. in rats. Int. J. Appl. Res. Nat. Prod. 2010;3(4):36–43. [Google Scholar]
- 191.Kim J.h., Koo Y.c., Hong C.O., Yang S.Y., Jun W., Lee K.W. Mutagenicity and oral toxicity studies of Terminalia chebula. Phytother Res. 2012;26(1):39–47. doi: 10.1002/ptr.3504. [DOI] [PubMed] [Google Scholar]
- 192.Rathore H. A study on the cytological effects of Myrobalan (fruit of Terminalia chebula) in Allium tests. Ethnobot. Leafl. 2006;2006(1):9. [Google Scholar]
- 193.Arora S., Brits E., Kaur S., Kaur K., Sohi R.S., Kumar S., Verschaeve L. Evaluation of genotoxicity of medicinal plant extracts by the comet and VITOTOX® tests. J. Environ. Pathol. Toxicol. Oncol. 2005;24(3) doi: 10.1615/jenvpathtoxoncol.v24.i3.50. [DOI] [PubMed] [Google Scholar]
- 194.Rathore H., Makwama M. Prevention of lead toxicity in Allium cepa root tip cells withmyrobalan (fruit of Terminalia chebula Retz.) Biochem. Cell. Arch. 2005;5(2):169–176. [Google Scholar]
- 195.Rathore H. Prevention of aluminium chloride-induced mitodepression with myrobalan (fruit of Terminalia chebula, Retz, Combretaceae) in Allium cepa model. Ethnobot. Leafl. 2006;2006(1):29. [Google Scholar]
- 196.Bag A., Bhattacharyya S.K., Chattopadhyay R.R. The development of Terminalia chebula Retz.(Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013;3(3):244–252. doi: 10.1016/S2221-1691(13)60059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Arseculeratne S.N., Gunatilaka A.L., Panabokke R.G. Studies on medicinal plants of Sri Lanka. Part 14: toxicity of some traditional medicinal herbs. J. Ethnopharmacol. 1985;13(3):323–335. doi: 10.1016/0378-8741(85)90078-9. [DOI] [PubMed] [Google Scholar]
- 198.Ponnusankar S., Pandit S., Venkatesh M., Bandyopadhyay A., Mukherjee P.K. Cytochrome P450 inhibition assay for standardized extract of Terminalia chebula Retz. Phytother Res. 2011;25(1):151–154. doi: 10.1002/ptr.2993. [DOI] [PubMed] [Google Scholar]
- 199.Sireeratawong S., Jaijoy K., Panunto W., Nanna U., Lertprasertsuke N., Soonthornchareonnon N. Acute and chronic toxicity studies of the water extract from dried fruits of Terminalia bellerica (Gaertn.) Roxb. In Spargue-Dawley rats. Afr. J. Tradit., Complementary Altern. Med. 2013;10(2):223–231. doi: 10.4314/ajtcam.v10i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.