Abstract
Several lines of evidence link deficient serotonin function and SUDEP. Chronic treatment with serotonin reuptake inhibitors (SRIs) reduces ictal central apnoea, a risk factor for SUDEP. Reduced medullary serotonergic neurones, modulators of respiration in response to hypercapnia, were reported in a SUDEP post‐mortem series. The amygdala and hippocampus have high serotonergic innervation and are functionally implicated in seizure‐related respiratory dysregulation. We explored serotonergic networks in mesial temporal lobe structures in a surgical and post‐mortem epilepsy series in relation to SUDEP risk. We stratified 75 temporal lobe epilepsy patients with hippocampal sclerosis (TLE/HS) into high (N = 16), medium (N = 11) and low risk (N = 48) groups for SUDEP based on generalised seizure frequency. We also included the amygdala in 35 post‐mortem cases, including SUDEP (N = 17), epilepsy controls (N = 10) and non‐epilepsy controls (N = 8). The immunohistochemistry labelling index (LI) and axonal length (AL) of serotonin transporter (SERT)‐positive axons were quantified in 13 regions of interest with image analysis. SERT LI was highest in amygdala and subiculum regions. In the surgical series, higher SERT LI was observed in high risk than low risk cases in the dentate gyrus, CA1 and subiculum (p < 0.05). In the post‐mortem cases higher SERT LI and AL was observed in the basal and accessory basal nuclei of the amygdala and peri‐amygdala cortex in SUDEP compared to epilepsy controls (p < 0.05). Patients on SRI showed higher SERT in the dentate gyrus (p < 0.005) and CA4 (p < 0.05) but there was no difference in patients with or without a psychiatric history. Higher SERT in hippocampal subfields in TLE/HS cases with SUDEP risk factors and higher amygdala SERT in post‐mortem SUDEP cases than epilepsy controls supports a role for altered serotonergic networks involving limbic regions in SUDEP. This may be of functional relevance through reduced 5‐HT availability.
Keywords: amygdala, hippocampus, serotonin transporter, SRI, SUDEP
1. INTRODUCTION
The mechanisms of sudden unexpected death in epilepsy (SUDEP) are uncertain. The main risk factor is poorly controlled generalised tonic–clonic seizures and, as many deaths occur peri‐ictally, during sleep or nocturnally, failure of arousal mechanisms interacting with respiratory autonomic regulation have been proposed, including serotonergic network dysfunction [1]. This is supported by experimental and clinical evidence [2]. Serotonin (5‐hydroxytryptamine [5‐HT]) can inhibit seizures and reduce seizure susceptibility and 5‐HT availability contributes to the anticonvulsant action of several common anti‐seizure medications [3]. Serotonergic brainstem neurones mediate brainstem arousal mechanisms and regulate medullary respiratory centres responsiveness to hypercapnia [4]; focal medullary atrophy [5, 6, 7], including reduced serotonergic neurones [8] is reported in SUDEP. Recent studies identified a lower incidence of ictal central apnoea in patients receiving serotonin reuptake inhibitors (SRIs) [9] and a reduction of SUDEP incidence in Dravet syndrome patients receiving fenfluramine, which increases serotonin levels [10]. SRIs act on the sodium‐dependent serotonin transporter (SERT or 5‐HT transporter [5‐HTT]) which is coded by the SLC6A4 gene and an integral membrane protein responsible for the specific reuptake of 5‐HT from the synaptic cleft, thereby regulating available 5‐HT and serotoninergic neurotransmission.
Mesial temporal lobe structures, including the amygdala, are also implicated in SUDEP. Stimulation and EEG studies have shown that seizure spread to amygdala and neighbouring limbic areas are associated with respiratory dysfunction [11, 12, 13] and ictal central apnoea is more common with temporal lobe onset than other seizures [14]. Altered amygdala volume has also been observed in SUDEP [15] and in neuropathological studies we reported deficient neuropeptide networks in SUDEP [16] and altered amygdala neuronal and glial adenosine receptors that associated with SUDEP risk [17]. These studies suggest that specific regional, seizure‐related cellular adaptations arise in SUDEP. As the amygdala is enriched in serotonergic afferents from the brainstem raphe nuclei [18] these networks may also be altered in SUDEP, contributing to post‐ictal period amygdala and autonomic dysfunction.
This study sought to quantify the relative distribution of SERT in a surgical series of temporal lobe epilepsy and hippocampal sclerosis (TLE/HS) cases in mesial temporal lobe regions, stratifying cases for SUDEP risk and considering any psychiatric history and SRI treatments. In addition, in a post‐mortem (PM) series we quantified SERT in specific amygdala subnuclei in SUDEP cases compared to control groups to test the hypothesis that serotonergic networks could be modified in SUDEP.
2. METHODS
2.1. Case selection
The cases were selected from the UCL Epilepsy Society brain and tissue bank. The project has ethical approval and patients provided written consent for research participation. The surgical cases were 75 adult patients who underwent temporal lobectomies (2009–2017) with a clinical and radiological diagnosis of hippocampal sclerosis (HS); cases were included where tissue samples were available from the temporal lobe, hippocampus body and amygdala. The neuropathology diagnosis was reviewed and HS subtype revised according to current ILAE criteria [19] the majority being type 1 HS. Clinical records were reviewed to extract data regarding seizure history, including age of onset, seizure types (generalised convulsive, focal) and frequency. Neuropsychiatric history and treatments were also independently reviewed by an experienced neuropsychiatrist (JF) regarding a diagnosis of pre or post‐operative depression or anxiety disorder as distinct from postictal mood disturbance, medication‐related mood disorders or epilepsy‐related psychosis (Table 1). In addition, any SRI anti‐depressive medications taken at the time of surgery or following surgery were noted.
TABLE 1.
Groups of TLE cases stratified according to risk of SUDEP
| Group | SUDEP RISK GROUP | Hippocampal Sclerosis Subtypes Type 1: Other type | Gender F:M (%) | Side L:R (%) | Age epilepsy onset Age at surgery/death (years, (SD)) | Focal seizures Without LOA With LOA (none: monthly: more frequently (% of cases) | Nocturnal Sz, episodes SE (% of cases) | Psychiatric history Pre‐op%, Post‐op %, SRI pre‐op N (%) |
|---|---|---|---|---|---|---|---|---|
| SURGICAL SERIES |
High Risk (HR) N = 16 |
62.5%:37.5% | 56.3:43.8 | 62.5:37.5 |
13.7 (11.3) 24.2 (10.3) |
0:27: 73 6.7:33.3:60 |
33% 20% |
31.3% 66.7% 2 (13%) |
|
Intermediate Risk (IR) N = 11 |
81.8%:18.2% | 63.6:36.4 | 63.6; 36.4 |
12.7 (7.1) 35 (15.4) |
0:44:56 0:40:60 |
36.4% 36.4% |
54.5% 45.5% 3 (27%) |
|
|
Low Risk (LR) N = 48 |
75%:25% | 50: 50 | 62.5:37.5 |
15 (11.8) 38.8 (15.1) |
17.4:19.6:63 0:17.4:82.6 |
8.3% 2.1% |
36.2% 47.9% 7 (15%) |
|
| Significance between groups | n/s | n/s | n/s |
n/s n/s |
n/s n/s |
p = 0.023 p = 0.002 |
n/s |
| CAUSE OF DEATH groups N = CASES (SECTIONS, Left and right) | No focal brain pathology/ lesion (%) | Gender F:M (%) | Side present L:R (side not categorised) (%) | Mean age at death (range) Years | Level of amygdala: Rostral:Mid:Caudal (% cases) | ROI Nuclei included in analysis (N) LAT: BASAL:ACC BASAL: PAC | Mean PMI Fixation Time Days (Range) | |
|---|---|---|---|---|---|---|---|---|
| PM SERIES | SUDEP N = 17 (27) | 55.6% | 59:41 | 37:41 (22) | 36.59 (16–56) | 59:26:15 | 27:22:22:22 |
3.09 (1–9) 74 (10–480) |
| EPILEPSY CONTROL GROUP N = 10 (17) | 41% | 59:41 | 24:35 (41) | 63.94 (44–85) | 53:29:18 | 16:17:16:15 |
3.62 (1–6) 32 (12–75) |
|
| NON EPILEPSY CONTROLS N = 8 (13) | 69.2% | 23:74 | 54:46 | 39.69 (23–69) | 54:15:31 | 12:13:13:12 |
5.1 (1–12) 55.8 (9–100) |
|
| Significance between groups | n/s | n/s | n/s | p < 0.0001 | n/s | n/s |
n/s n/s |
Note: The frequency of GCS was used as the criterion to stratify groups. Hippocampal sclerosis subtypes other = type 3, no HS and cases with uncertain HS types (incomplete subfields represented in the resected specimen).
Abbreviations: GCS, generalised seizures; IPI, initial precipitating injury; LOA, loss of awareness; n/s, not significant; PMI, post‐mortem interval; pre‐op, pre‐operatively; post‐op, post operatively; Sz, seizures; SE, status epilepticus; SRI, serotonin reuptake inhibitor. Kruskall Wallis test was used to compare groups.
Risk stratification for SUDEP was based on the number of generalised convulsive seizures (GCS) in the previous 12 months at the last pre‐operative assessment [20, 21] in line with risk criteria used in published MRI studies [22, 23] and as detailed in a recent pathology study [17]. Among our patients, there were 16 high risk (≥3 GCS), 48 low risk (no GCS) and 11 intermediate risk (1–2 GCS). Between these three risk groups there were no significant differences in the ILAE HS subtypes, gender, age of onset, age at surgery, frequency of focal seizures types or psychiatric history (Table 1). There was a lower frequency of nocturnal seizures (p = 0.02) and episodes of status epilepticus (p = 0.002) in low risk compared to intermediate and high risk groups (Table 1).
The amygdala has rich serotonergic innervation and high SERT levels [18, 24]. Since surgical specimens are limited by fragmentation and incomplete anatomical representation of all amygdala nuclei, we also used PM epilepsy cases to investigate this region. We included amygdala from a series of 17 epilepsy cases with SUDEP as well as 10 epilepsy cases without SUDEP (epilepsy controls) and 8 non‐epilepsy controls, collected between 2006 to 2017. In 14 cases in the SUDEP group, recent seizure(s) with recovery was reported in the 24‐h period prior to death; other clinical and neuropathology data is summarised in Table 1 (detailed in Table S1).
2.2. Tissue preparation, immunohistochemistry and analysis
From each surgical case, three tissue samples from the temporal lobe (~2.5 cm posterior to the temporal pole), the hippocampus body and amygdala were selected. Serial sections were cut at 5 microns thickness and immunohistochemistry for SERT (1:50,000 Millipore MAB5618) and NeuN (Millipore, 1: 2000) carried out using standard methods (detailed in Supplementary files). The slides were scanned at 40x magnification using a Zeiss AxioScan Zen 2.3 slide scanner. Nine regions of interest (ROI) were defined using Definiens Tissue Studio (Definiens AG, Munich, Germany) image analysis system by one observer (AC) blinded to the SUDEP risk group; placement of all ROI was then validated by a second person (SP) and were as follows (Figure S1a, b): Three Temporal lobe ROI: Superficial cortex (Layer I of the middle temporal gyrus), Deeper cortex (Layer II to VI of the middle temporal gyrus) and subcortical white matter. Five Hippocampal ROI: dentate gyrus including the granule cell layer and molecular layer, CA4 subfield of hippocampus including the region between the blades of the dentate gyrus, CA1 subfield of hippocampus including the maximal representation of pyramidal cell layer but excluding CA2, Subiculum including its maximal representation on section and hippocampal white matter the stratum radiatum, lacunosum and moleculare between CA1 pyramidal cell layer and the dentate gyrus. Amygdala regions in surgical samples were outlined based on the cytoarchitecture on NeuN, excluding any white matter and neocortex; due to the fragmented nature of amygdala samples in surgical material it was not possible to further delineate subnuclei. Using Definiens Tissue Studio software a high intensity threshold was set for the chromogen detection for each antibody which was applied across all cases (Figure S1c, d) and the total labelling index (LI) (percentage area of cellular immunostaining) for each ROI was measured.
For the PM series, in 22/35 cases FFPE samples from both left and right amygdala were available and from one side only in the remainder (Table 1). Sections were cut at 5 micron thickness and one section was stained with LFB/CV for anatomical orientation and delineation of the main human amygdala subnuclei [25]. The anatomical level of the amygdala was categorised as one of three coronal levels in a rostro‐caudal direction: rostral amygdala (anterior to lateral ventricle) (Figure S1g), mid amygdala (at level of temporal horn)(Figure S1h) and caudal amygdala (level of anterior hippocampus)(Figure S1i) as previously described [16]. The central nucleus was not represented in all coronal levels and therefore not included in the analysis. Double immunofluorescence labelling for myelin basic protein (SMI94) with SERT was carried using standard methods (see supplemental methods file for detail) and the slides scanned on Zeiss AxioScan Zen 2.3 slide scanner. Using Zen (Carl Zeiss) software the lateral, basal, accessory basal and periamygdala cortex (PAC) subnuclei were identified using the SMI94 channel only for placement of ROI (Figure S1h) with three to five ROI were randomly placed per subnuclei (Figure 1B) to cover a total area from 6.3 × 105 μm2 in the basal to 11.3 × 105 μm2 in the lateral nucleus. This sampling protocol was established in a pilot series of 5 cases with good reproducibility and intra class correlation coefficients of 0.97 to 0.7 for the four subnuclei. A high threshold for detection of SERT labelling was set and kept constant across ROI and cases; both the mean LI and total length of SERT‐positive axons (AL) per area for each region was calculated.
FIGURE 1.
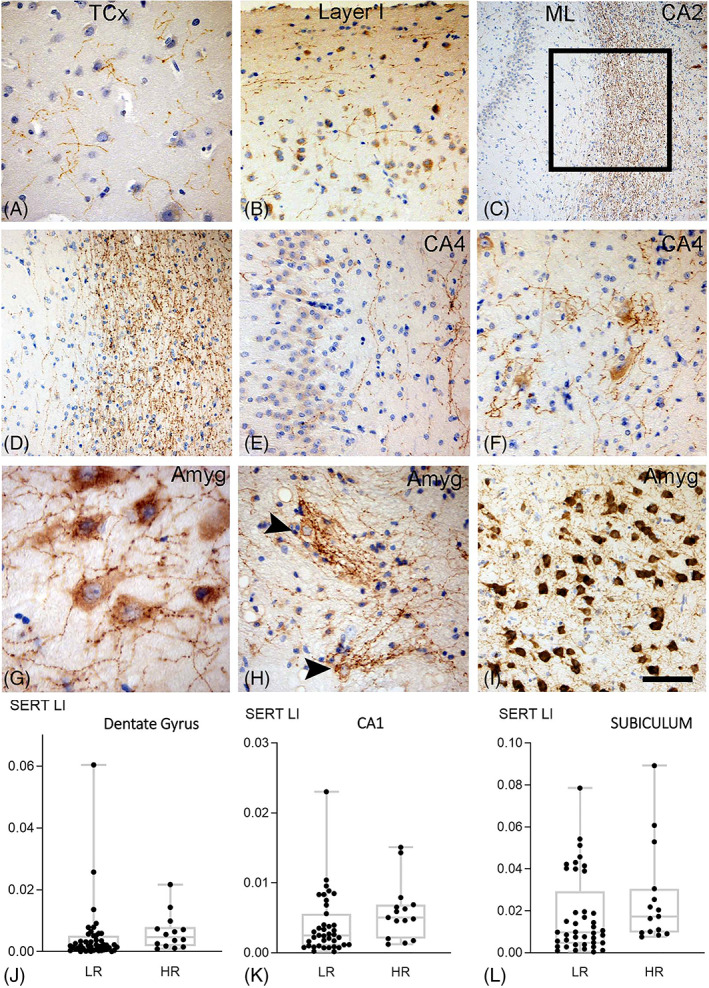
SERT in TLE surgical series with hippocampal sclerosis. (A) Fine axonal varicose SERT networks were present in temporal neorcortex (TCx). (B) There was an impression of condensation of SERT‐positive fibres in the superficial cortical layers (Layer I). (C) Dense SERT axonal networks were also present in the hippocampal white matter (stratum radiatum, lacunosum and moleculare), shown here between the molecular layer (ML) of the dentate gyrus and the pyramidal cell layer of CA2 (box shown at higher magnification in (D)) (E) SERT axons were also present in the dentate gyrus and CA4 region. (F) In CA4 ‘nets’ of SERT positive processess surrounded pyramidal neurones. (G) The amygadala (Amyg) was enriched in SERT with numerous beaded axons in proximity to neurones. (H) SERT positive neurones with complexed tuft like branches were also present in the amygdala (Amyg). (I) Amygdala regions with intense SERT positivity were observed. Scatter plots of mean SERT labelling index (LI) in high risk (HR) compared to and low risk (LR) for SUDEP cases which showed a significant increase in the high risk group in the (J) dentate gyrus, (K) CA1 and (L) subiculum regions. Bar in I equivalent to approx. 50 microns in A, B. D. E. F, H, I, 150 microns in C and 35 microns in H
2.3. Expression of SLC64A in TLE datasets
We also explored SLC64A gene expression in existing related temporal lobe epilepsy surgical datasets in order to ascertain if this reflected the differences observed with immunohistochemistry. The final differential expression outputs from two RNA Seq experiments were provided by the data generators on request [26, 27]. Dataset 1 was a comparison between TLE and control cortex (TLE, N = 6; Control, N = 5) and hippocampus (TLE, N = 6; Control, N = 16). Dataset 2 assessed the same brain regions of TLE patients but stratified into high risk (cortex, N = 2; hippocampus, N = 8) and low risk (cortex, N = 3; hippocampus, N = 4) based on prolonged (>50 s) postictal generalized EEG suppression (PGES), regarded as a risk factor for SUDEP. The expression of SLC64A was extracted from each dataset for further analysis. For the full details on RNA Seq protocols used, sample sets and differential expression analysis we refer to the relevant papers.
2.4. Statistical analysis
Statistical analysis was carried out with SPSS (version 22, IBM) for non‐parametric tests to compare data between cause of groups (Kruskall Wallis and Mann–Whitney tests), regions within groups (Friedman test) and clinical correlations (Spearman's rho test); p values of <0.05 were regarded as significant and corrected for multiple comparisons as detailed in the results. For the PM studies Wilcoxon test was used to compare SERT between subnuclei within cause of death groups. For graphical representation of data, GraphPad Prism 9 (University of California, San Diego) was used.
3. RESULTS
3.1. Qualitative description
SERT labelling in surgical temporal lobectomies identified networks of beaded and varicose axons of variable length, like previous descriptions [24, 28]. In the temporal lobe, SERT‐positive axons were identified through the cortex layers (Figure 1A), noticeably in the cell poor cortical layer I, running parallel with the cortical surface (Figure 1B), whereas the subcortical white matter was traversed by less frequent long axons. In the hippocampus, dense networks of SERT‐positive axons running in the stratum lacunosum/moleculare was a prominent finding (Figure 1C, D) with less dense axons in the molecular and granule cell layer of the dentate gyrus (Figure 1C–E). Proximity of SERT‐positive axons to remaining hippocampal pyramidal cells was noted, in some cases forming dense net‐like arrangements around CA4 neurones (Figure 1F); occasional neurones in CA2 and CA1 showed weak cytoplasmic labelling. In the subiculum there were similar positive processes running through pyramidal cell layer and focal neuronal labelling was noted. In the amygdala, focally dense networks of axons were seen traversing across (Figure 1G) or enmeshed around labelled neurones (Figure 1H, Figure 1I).
In the PM amygdala samples, SERT‐positive meshworks of beaded and varicose axons of varying densities were highlighted in all amygdala subnuclei and throughout the rostro‐caudal levels examined (Figure 2A). In addition, short, compact bundles of SERT labelled axons, particularly in the lateral and basal nuclei, were seen which appeared myelinated. The paralaminar region adjacent to the lateral ventricle showed prominent aggregates of SERT‐positive fibres (Figure 2B). In the PAC, SERT neuronal cytoplasmic labelling was also noted in addition to extensive mainly unmyelinated axonal networks (Figure 2C). Occasional SERT‐positive neurones were also noted in the basal and accessory basal nucleus.
FIGURE 2.
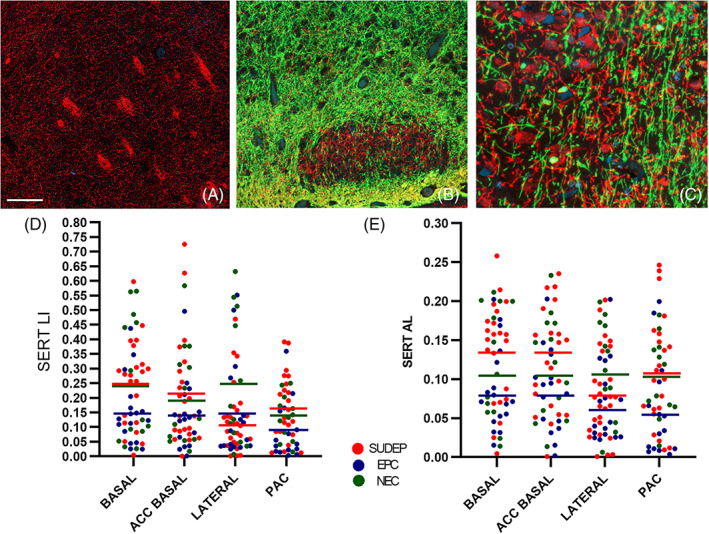
SERT (red channel) combined with myelin basic protein (SMI94) labelling (green channel) in post‐mortem amygdala. (A) Prominent meshworks of SERT‐positive fibres were present in the lateral nucleus with some condensation in bundles (B). The paralaminar nucleus was highlighted by a relative lack of myelin but SERT‐positive axons. (C) Shows the region of PAC at higher magnification with dense bundles of SERT‐positive unmyelinated axons distinct from other myelinated fibres and neurones. (D) Scatter graphs of SERT labelling index (LI) and (E) axon length / area (AL) in amygdala regions in the post‐mortem cases. The bars are the mean values for each region in the cause of death groups (SUDEP in red, Epilepsy controls (EPC) in blue and Non‐epilepsy controls (NEC) in green). There were significant differences between subnuclei noted in SUDEP group with relative lower SERT in the lateral nucleus (Wilcoxon rank test, p ≤ 0.001) and in the epilepsy controls lower SERT in the PAC (Wilcoxon rank test, p < 0.05) but there were no significant differences between subnuclei in non‐epilepsy controls. Between cause of death groups, higher SERT LI was seen in SUDEP than epilepsy control group in the basal nucleus and PAC (p < 0.05) and length of SERT‐positive axons in the basal, accessory basal nucleus and PAC (p < 0.05). The length of SERT‐positive processes was significantly lower in epilepsy controls than non‐epilepsy controls in the lateral nucleus and PAC regions (p < 0.05). Bar equivalent to approx. 200 microns in A, 100 microns in B and 50 microns in C
3.2. Quantitative analysis
3.2.1. SERT in surgical series: Clinical and psychiatric correlations
There was significant variation in SERT labelling between all ROI with highest labelling in the amygdala and subiculum (p < 0.0001) (Table 2). For all surgical cases there was no significant correlation between SERT LI and age at surgery, gender, side of HS, history of status epilepticus, nocturnal seizures or frequency of focal seizures with loss of awareness. Higher SERT was identified in the hippocampus white matter in patients with a history of GCS compared to those without (p = 0.01) and significantly lower superficial cortical SERT LI was observed in patients with more frequent focal seizures with preserved awareness (p = 0.04). There was a positive correlation between age of epilepsy onset and SERT LI in CA1 region (p = 0.039). There were significant differences in SERT in CA4 between the subtypes of HS (p = 0.013) but not in other regions.
TABLE 2.
Quantitative analysis of the SERT regional labelling in epilepsy surgical and post mortem (amygdala only) cases
| Surgical groups | Cortex Supf. Cortex deep LI mean (SD) | WM T lobe WM hippo LI mean (SD) | CA4 CA1 LI mean (SD) | DG SUBIC LI mean (SD) | AMYG LI * mean (SD) |
|---|---|---|---|---|---|
| High risk |
0.28 (0.27) 0.29 (0.3) |
0.11 (0.15) 0.822 (0.15) |
0.48 (0.39) 0.58 (0.41) |
0.62 (0.58) 2.6 (2.36) |
2.04 (2.34) |
| Medium risk |
0.33 (0.22) 0.26 (0.13) |
0.07 (0.04) 0.89 (0.78) |
0.85 (0.93) 0.72 (0.65) |
1.06 (1.83) 1.99 (2.6) |
4.23 (3.79) |
| Low risk |
0.33 (0.26) 0.37 (0.39) |
0.09 (0.16) 0.88 (1.2) |
0.45 (0.97) 0.39 (0.42) |
0.44 (0.94) 1.77 (1.87) |
2.69 (3.9) |
| Post Mortem Groups | Lateral Nucleus LI (SD) Axonal length μm/μm2(SD) | Basal Nucleus LI (SD) Axonal length μm /μm2(SD) | Accessory Basal Nucleus LI (SD) Axonal length μm /μm2(SD) | PAC Nucleus LI (SD) Axonal length μm /μm2(SD) | |
|---|---|---|---|---|---|
| SUDEP |
0.12 (0.11) 0.08 (0.05) |
0.25 (0.14) 0.13 (0.065) |
0.21 (0.18) 0.13 (0.08) |
0.16 (0.11) 0.11 (0.07) |
|
| Epilepsy controls |
0.14 (0.16) 0.069 (0.05) |
0.15 (0.11) 0.08 (0.054) |
0.14 (0.12) 0.08 (0.05) |
0.085 (0.09) 0.058 (0.063) |
|
| Non Epilepsy controls |
0.24 (0.22) 0.11 (0.063) |
0.25 (0.21) 0.11 (0.073) |
0.20 (0.17) 0.11 (0.06) |
0.13 (0.076) 0.10 (0.05) |
Note: LI = Labelling index shown as a percentage in surgical cases; post‐mortem data shown as both a labelling index and the total length of axons per unit area (shown in microns/micron2). The quantification for surgical and post mortem cases was obtained using two different image analysis systems and immunohistochemistry methods (see text for further details).
Abbreviations: Amyg, amygdala; DG, dentate gyrus; Hippo, hippocampus; PAC, peri‐amygdala cortex; SUBIC, subiculum; Supf., superficial cortex (layer I); T lobe, temporal lobe; WM, white matter.
Patients with and without a psychiatric history had similar SERT LI in all regions. Patients with a history of depression or anxiety pre‐ or post‐operatively had similar findings as those without this history. Twelve patients were taking SRI medication at the time of surgery (none were receiving tricyclic antidepressants), represented in all risk groups (Table 1); significantly higher SERT labelling was observed in the DG (p = 0.004) and CA4 (p = 0.04) in patients taking SRI at time of surgery.
3.2.2. SERT in surgical series: SUDEP Risk
Significantly higher SERT LI was observed in high risk compared to low risk for SUDEP cases in the DG (p = 0.024), CA1 (p = 0.042) and subiculum (p = 0.043) (Figure 1J,K,l). When patients taking SRIs were excluded these differences were still significant. There were significant positive correlations between SERT and neuronal (NeuN) LI in the temporal lobe cortex, CA1, CA4, subiculum and amygdala (p < 0.05 to <0.0001) in the low risk SUDEP group. Correlations were less significant in the high risk group and observed only in in CA1, CA4 and amygdala (p < 0.05) but not in the cortex or subiculum. Neither low or high risk cases showed a correlation between SERT and NeuN in the DG region.
3.2.3. SERT in post‐mortem series: Amygdala subnuclei
There was significant variation in SERT expression between subnuclei in the SUDEP groups with highest LI and AL in the basal nucleus and lowest measures in the lateral nucleus (p = 0.001). Significant variation was also noted in the epilepsy controls but with lowest LI and AL in the PAC (p < 0.05). There were no significant differences between subnuclei in the non‐epilepsy controls (Table 2, Figure 2d, e).
Between cause of death groups, SERT was significantly higher in SUDEP than epilepsy controls for both LI and AL in the basal nucleus and PAC (p < 0.05) and for AL only in the accessory basal nucleus (p < 0.05). SERT AL was significantly lower in epilepsy controls than non‐epilepsy controls in the lateral nucleus and PAC (p < 0.05). There were no significant differences between SUDEP and non‐epilepsy controls (Figure 2d, e).
From available drug histories, six patients were on SRIs at the time of death, 4 in the SUDEP, 2 in the non‐epilepsy control group (Table S1); there was incomplete or limited drug and psychiatric information in some cases. Non‐significantly higher SERT LI and AL were seen in all amygdala regions in patients on SRIs than those not. There was no correlation between SERT labelling and age at death, a history of a recent seizure in the 24 h period prior to death or whether there was an underlying neuropathology lesion identified at post‐mortem (see Table S1). There was no difference in SERT measurements in subnuclei relative to the coronal level of amygdala or between left and right sides in any cause of death group. Higher SERT LI were present in the lateral and basal nuclei in females compared to males (p < 0.005, p < 0.05). There was no significant difference in post‐mortem intervals (PMI) and fixation times between the cause of death groups (Table 1) and SERT measurements were not significantly reduced with longer PMI or fixation times.
3.3. Gene expression data analysis
Analysis of SLC6A4 gene expression in TLE datasets compared to controls showed a non‐significant up‐regulation of SLC6A4 (positive fold‐change) in the cortex and in the hippocampus a non‐significant down‐regulation of SLC6A4 (negative fold‐change); however the overall mean base (normalised counts, value of expression) was low in the epilepsy hippocampi and this does not rule out higher cell‐type specific expression. In datasets comparing the same brain regions in high SUDEP risk compared to low SUDEP risk TLE cases a log fold increase in SLC64A of 1.66 (absolute fold‐change of 3) in the high‐risk group was seen but again was not significantly different.
4. DISCUSSION
Clinical and experimental data implicate the serotonergic system in SUDEP although supportive neuropathological evidence is limited. In a group of surgical patients with TLE/HS we identified increased hippocampal SERT labelling in a high SUDEP risk group with overall highest SERT labelling in the amygdala and subiculum. In amygdala from SUDEP PM cases we showed significantly higher SERT in basal, accessory basal and PAC regions compared to an epilepsy control group. SRI treatment was associated with increased SERT expression suggesting that both drug treatment and seizures can modulate SERT. Higher SERT expression in mesial temporal lobe regions with SUDEP risk could have functional implications through reduced available 5‐HT in the vulnerable post‐ictal period.
4.1. SERT regional distribution in SUDEP
5‐HT synthesised in the brainstem raphe nuclei provide extensive projections throughout the neuro‐axis. SERT immunohistochemistry provides a specific tissue marker to highlight and quantify serotonergic afferent networks in different regions [29]. In surgical cases of TLS/HS, observations of higher SERT levels in the subiculum than hippocampus proper and prominent axons in the stratum radiatum, lacunosum and moleculare are in line with findings in animals [30]. In the high risk SUDEP group we observed greater SERT in the subiculum, CA1 and DG compared to the low risk group. The subiculum is a major output centre of the hippocampus, a putative zone for spontaneous seizure‐like activity in TLE/HS [31] with experimental evidence that 5‐HT regulates firing of subicular principal neurones [32]. The subiculum included in this study was the pro‐subiculum, adjacent to CA1 and known to have the densest serotonergic innervation with efferent projections to the amygdala [33]. Altered available synaptic serotonin could theoretically modulate subicular function, relevant to seizure initiation and spread, and potentiate SUDEP risk; however this hypothesis requires validation that the increased SERT reflects functional, membrane‐bound transporters.
The amygdala has dense serotonergic innervation across species [29]. In a study of 232 cases using positron emission tomography (PET) and SERT ligand 11C‐DASB, high receptor levels were observed in the amygdala compared to hippocampal and cortical regions [18]. We confirmed overall high SERT in the amygdala region in TLE/HS cases but the surgical samples have incomplete representation, limiting comparison between SUDEP risk groups. We therefore carried out further regional analysis in a PM series. A recent stereological study of normal amygdala showed lower SERT axon density in the lateral nucleus although with no significant regional differences [24]. Our control group had no significant regional variation in SERT axons and prominent labelling in the paralaminar nucleus, also consistent with anatomical studies [34]. In epilepsy cases, however, significant regional variation in SERT was observed, with significantly higher levels in SUDEP than the epilepsy controls group which were, in turn, lower in regions than non‐epilepsy controls. In SUDEP, significantly higher SERT was noted in the basal, accessory basal and PAC regions. Of note, in a recent paediatric study of eight patients, stimulation‐induced apnoea was localized to a region encompassing the basal lateral, basomedial and cortical‐medial amygdala region [13] which correlates with the anatomical basal, accessory basal and PAC regions in the current study; this could implicate serotonergic networks in amygdala‐induced apnoea. Our current findings also contrast with our previous study of amygdala neuropeptides (NPY, somatostatin and galanin) using the same PM cohort, where significantly higher levels were present in epilepsy controls than SUDEP cases [16]. This suggests that specific patterns of amygdala cellular network reorganisation in epilepsy, characterises SUDEP.
4.2. Evidence for seizure‐related modulation of SERT
Seizures can modulate serotonergic networks, including 5‐HT receptors and SERT [3]). In a TLE/HS surgical series reduced SERT receptor binding was observed in temporal neocortex compared to controls [35]. We also noted significantly lower superficial cortical SERT in patients with more frequent focal seizures without loss of awareness but higher SERT in the hippocampal white matter in patients with GCS, suggesting modulation could be seizure‐type as well regionally specific. A TLE study showed lower 5‐HT levels in TLE/HS cases with histories of generalised seizures [36], in keeping with higher SERT activity and GCS as SUDEP risk factors [1].
Serotonergic axons can regenerate following injury and exhibit compensatory sprouting from non‐injured axons, therefore demonstrating a unique potential for repair and remodelling [37]. Indeed, we noted net‐like bundles of axons in relation to neurones, particularly in CA4 in hippocampal sclerosis and dense SERT expression in relation to neurones, particularly in the PAC and subiculum. Transient ‘5‐HT‐absorbing neurones’ have been recognised in the developing thalamus and limbic cortex, that express SERT, enabling 5‐HT re‐uptake from the extracellular space although unable to synthesise it (i.e. tryptophan hydrogenase negative) [38, 39, 40]. In the serotonergic brain atlas study no association was shown between SERT receptor binding and regional mRNA levels arguing against local neuronal expression [18] and analysis of data from the Allen Human Brain Atlas confirms the highest levels for supratentorial SERT mRNA is in the amygdala [41]. Analysis of gene expression datasets in TLE cohorts confirmed low SERT expression levels in mesial temporal brain regions, but with mild non‐significant upregulation in TLE and including cases at higher risk for SUDEP.
4.3. The effect of SRI drug history
Deficient 5‐HT activity may underlie or contribute to the reciprocal relationship between epilepsy and depression [42, 43]. A meta‐analysis of studies in major depression, including post‐mortem based studies, confirms reduced limbic region and amygdala SERT [44]. One 11C DASB PET study also showed SERT reduction in patients with TLE and depression compared to TLE alone [43]. We did not identify any differences in SERT labelling in TLS/HS patients with neuropsychiatric illness compared to those without; this study however, may be insufficiently powered to investigate this or could suggest other neurotransmitter involvement, as indicated in experimental models [45]. However, in patients receiving SRI treatment we identified higher SERT in hippocampal and amygdala regions. SERT receptor occupancy is higher in the amygdala than cortical regions following SRI treatment [46]; thus, increased SERT expression may represent an adaptive upregulation of functioning receptor rather than enhanced internalization.
4.4. Limitations of this study
There are several limitations to this study. In the surgical series we did not include a non‐epilepsy control group as normal surgical hippocampal tissue is not available. Our risk stratification was based on a single, albeit strong risk factor (GCS frequency) but dependent on the accuracy of the clinical records. Nevertheless, the advantages of surgical tissues is the uniformity of the underlying pathology, identical regions of interest can be studied and fixation times are short. In the post‐mortem series the SUDEP cases had a lower mean age of death than the epilepsy group; although we noted no significant age‐related differences this could be a potential limitation. Serotonin transporter (SERT) remains relatively stable in post‐mortem tissues (Verney et al., 2002) and in this series we did not observe effects in relation to fixation time. In the PM series we did not have full drug history (including SRIs) in all cases. Importantly, we lacked genetic data for SERT variants [47], promotor polymorphisms [48] or epigenetic alterations including methylation [49] which may regulate SERT expression [50]. The short versus long isoform of the promoter region of the SLC6A4 gene which codes SERT has been associated with lower transcription rates, SERT function and increased amygdala activity [51] although this finding is not consistent across studies [50, 52], one post‐mortem study showing no difference in SERT expression in relation to polymorphisms [53]. Further, SERT immunohistochemistry measures total levels only but not cellular localisation or functioning membrane receptors which require different experimental strategies.
In summary, using quantitative immunohistochemistry we detected increased SERT in limbic regions in association with SUDEP risk. Further study of how this could translate to dysfunctional neuronal serotonergic networks and lead to therapeutic prevention strategies for SUDEP is warranted.
AUTHOR CONTRIBUTIONS
SP, AC, BP, MO provided the pathology sample preparation and data collection; AS was involved in sample preparation; MG, BD, OD, JF provided clinical data; JL JDM provided the genetic correlations and data analysis; MT SP the study design and data analysis; all co‐authors in manuscript preparation and review.
Supporting information
Figure 1
Table 1
Appendix S1
ACKNOWLEDGEMENTS
This is an ethically approved project and supported through the National Institute of Neurological Disorders And Stroke of the National Institutes of Health (Award Numbers neuropathology of SUDEP: 5U01NS090415 and SUDEP admin core grant: U01‐NS090405). Epilepsy Society through the Katy Baggott Foundation, supports the UCL Epilepsy Society Brain and Tissue Bank. We are very grateful to Anita‐Beatrix Zborovschi in the brain bank for all her help with this project and Matt Ellis for his advice on the image analysis methods. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The authors have no conflicts of interest to declare and confirm this work upholds the rigorous ethical standards of the journal. All data is presented in this paper and supplemental files.
Patodia S, Somani A, Liu J, Cattaneo A, Paradiso B, Garcia M, et al. Serotonin transporter in the temporal lobe, hippocampus and amygdala in SUDEP . Brain Pathology. 2022;32:e13074. 10.1111/bpa.13074
DATA AVAILABILITY STATEMENT
All data is presented in this paper and supplemental files.
REFERENCES
- 1. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075–88. [DOI] [PubMed] [Google Scholar]
- 2. Zhan Q, Buchanan GF, Motelow JE, Andrews J, Vitkovskiy P, Chen WC, et al. Impaired serotonergic brainstem function during and after seizures. J Neurosci. 2016;36(9):2711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petrucci AN, Joyal KG, Purnell BS, Buchanan GF. Serotonin and sudden unexpected death in epilepsy. Exp Neurol. 2020;325:113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA. 2010;107(37):16354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mueller SG, Bateman LM, Laxer KD. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. Neuroimage Clin. 2014;5:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mueller SG, Nei M, Bateman LM, Knowlton R, Laxer KD, Friedman D, et al. Brainstem network disruption: a pathway to sudden unexplained death in epilepsy? Hum Brain Mapp. 2018;39:4820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patodia S, Tachrount M, Somani A, Scheffer I, Yousry T, Golay X, et al. MRI and pathology correlations in the medulla in sudden unexpected death in epilepsy (SUDEP): a postmortem study. Neuropathol Appl Neurobiol. 2020;47:157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patodia S, Somani A, O'Hare M, Venkateswaran R, Liu J, Michalak Z, et al. The ventrolateral medulla and medullary raphe in sudden unexpected death in epilepsy. Brain. 2018;141(6):1719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lacuey, N. , Martins R., Vilella L., Hampson J. P., Rani M. R. S., Strohl K., Zaremba A., Hampson J. S., Sainju R. K., Friedman D., Nei M., Scott C., Gehlbach B. K., Hupp N. J., Schuele S., Ogren J., Harper R. M., Allen L., Diehl B., Bateman L. M., Devinsky O., Richerson G. B. and Lhatoo S. (2019). "The association of serotonin reuptake inhibitors and benzodiazepines with ictal central apnea." Epilepsy Behav 98(Pt A): 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cross JH, Galer BS, Gil‐Nagel A, Devinsky O, Ceulemans B, Lagae L, et al. Impact of fenfluramine on the expected SUDEP mortality rates in patients with Dravet syndrome. Seizure. 2021;93:154–9. [DOI] [PubMed] [Google Scholar]
- 11. Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci. 2015;35(28):10281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lacuey N, Zonjy B, Londono L, Lhatoo SD. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology. 2017;88(7):701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhone AE, Kovach CK, Harmata GI, Sullivan AW, Tranel D, Ciliberto MA, et al. A human amygdala site that inhibits respiration and elicits apnea in pediatric epilepsy. JCI Insight. 2020;5(6):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lacuey N, Zonjy B, Hampson JP, Rani MRS, Zaremba A, Sainju RK, et al. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia. 2018;59(3):573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allen LA, Harper RM, Lhatoo S, Lemieux L, Diehl B. Neuroimaging of sudden unexpected death in epilepsy (SUDEP): insights from structural and resting‐state functional MRI studies. Front Neurol. 2019;10:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Somani A, Perry C, Patodia S, Michalak Z, Ellis M, Sisodiya SM, et al. Neuropeptide depletion in the amygdala in sudden unexpected death in epilepsy: a postmortem study. Epilepsia. 2020;61(2):310–8. [DOI] [PubMed] [Google Scholar]
- 17. Patodia S, Paradiso B, Garcia M, Ellis M, Diehl B, Thom M, et al. Adenosine kinase and adenosine receptors A1 R and A2A R in temporal lobe epilepsy and hippocampal sclerosis and association with risk factors for SUDEP. Epilepsia. 2020;61(4):787–97. [DOI] [PubMed] [Google Scholar]
- 18. Beliveau V, Ganz M, Feng L, Ozenne B, Hojgaard L, Fisher PM, et al. A high‐resolution in vivo atlas of the human Brain's serotonin system. J Neurosci. 2017;37(1):120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blumcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE commission on diagnostic methods. Epilepsia. 2013;54(7):1315–29. [DOI] [PubMed] [Google Scholar]
- 20. DeGiorgio CM, Curtis A, Hertling D, Moseley BD. Sudden unexpected death in epilepsy: risk factors, biomarkers, and prevention. Acta Neurol Scand. 2019;139(3):220–30. [DOI] [PubMed] [Google Scholar]
- 21. DeGiorgio CM, Markovic D, Mazumder R, Moseley BD. Ranking the leading risk factors for sudden unexpected death in epilepsy. Front Neurol. 2017;8:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allen LA, Harper RM, Kumar R, Guye M, Ogren JA, Lhatoo SD, et al. Dysfunctional brain networking among autonomic regulatory structures in temporal lobe epilepsy patients at high risk of sudden unexpected death in epilepsy. Front Neurol. 2017;8:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wandschneider B, Koepp M, Scott C, Micallef C, Balestrini S, Sisodiya SM, et al. Structural imaging biomarkers of sudden unexpected death in epilepsy. Brain. 2015;138(Pt 10):2907–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lew CH, Hanson KL, Groeniger KM, Greiner D, Cuevas D, Hrvoj‐Mihic B, et al. Serotonergic innervation of the human amygdala and evolutionary implications. Am J Phys Anthropol. 2019;170(3):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sorvari H, Soininen H, Pitkanen A. Calbindin‐D28K‐immunoreactive cells and fibres in the human amygdaloid complex. Neuroscience. 1996;75(2):421–43. [DOI] [PubMed] [Google Scholar]
- 26. Leitner DF, Mills JD, Pires G, Faustin A, Drummond E, Kanshin E, et al. Proteomics and Transcriptomics of the hippocampus and cortex in SUDEP and high‐risk SUDEP patients. Neurology. 2021;96(21):e2639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mills JD, van Vliet EA, Chen BJ, Janitz M, Anink JJ, Baayen JC, et al. Coding and non‐coding transcriptome of mesial temporal lobe epilepsy: critical role of small non‐coding RNAs. Neurobiol Dis. 2020;134:104612. [DOI] [PubMed] [Google Scholar]
- 28. Noristani HN, Olabarria M, Verkhratsky A, Rodriguez JJ. Serotonin fibre sprouting and increase in serotonin transporter immunoreactivity in the CA1 area of hippocampus in a triple transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2010;32(1):71–9. [DOI] [PubMed] [Google Scholar]
- 29. Asan E, Steinke M, Lesch KP. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem Cell Biol. 2013;139(6):785–813. [DOI] [PubMed] [Google Scholar]
- 30. Oleskevich S, Descarries L. Quantified distribution of the serotonin innervation in adult rat hippocampus. Neuroscience. 1990;34(1):19–33. [DOI] [PubMed] [Google Scholar]
- 31. Reyes‐Garcia SZ, Scorza CA, Araujo NS, Ortiz‐Villatoro NN, Jardim AP, Centeno R, et al. Different patterns of epileptiform‐like activity are generated in the sclerotic hippocampus from patients with drug‐resistant temporal lobe epilepsy. Sci Rep. 2018;8(1):7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petersen AV, Jensen CS, Crepel V, Falkerslev M, Perrier JF. Serotonin regulates the firing of principal cells of the subiculum by inhibiting a T‐type ca(2+) current. Front Cell Neurosci. 2017;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ding SL. Comparative anatomy of the prosubiculum, subiculum, presubiculum, postsubiculum, and parasubiculum in human, monkey, and rodent. J Comp Neurol. 2013;521(18):4145–62. [DOI] [PubMed] [Google Scholar]
- 34. de Campo DM, Fudge JL. Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci Biobehav Rev. 2012;36(1):520–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rocha L, Lorigados‐Pedre L, Orozco‐Suarez S, Morales‐Chacon L, Alonso‐Vanegas M, Garcia‐Maeso I, et al. Autoradiography reveals selective changes in serotonin binding in neocortex of patients with temporal lobe epilepsy. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1208–18. [DOI] [PubMed] [Google Scholar]
- 36. da Fonseca NC, Joaquim HP, Talib LL, de Vincentiis S, Gattaz WF, Valente KD. Hippocampal serotonin depletion is related to the presence of generalized tonic‐clonic seizures, but not to psychiatric disorders in patients with temporal lobe epilepsy. Epilepsy Res. 2015;111:18–25. [DOI] [PubMed] [Google Scholar]
- 37. Kajstura TJ, Dougherty SE, Linden DJ. Serotonin axons in the neocortex of the adult female mouse regrow after traumatic brain injury. J Neurosci Res. 2018;96(4):512–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen X, Petit EI, Dobrenis K, Sze JY. Spatiotemporal SERT expression in cortical map development. Neurochem Int. 2016;98:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–12. [DOI] [PubMed] [Google Scholar]
- 40. Jafari G, Xie Y, Kullyev A, Liang B, Sze JY. Regulation of extrasynaptic 5‐HT by serotonin reuptake transporter function in 5‐HT‐absorbing neurons underscores adaptation behavior in Caenorhabditis elegans. J Neurosci. 2011;31(24):8948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Komorowski A, James GM, Philippe C, Gryglewski G, Bauer A, Hienert M, et al. Association of Protein Distribution and Gene Expression Revealed by PET and post‐mortem quantification in the serotonergic system of the human brain. Cereb Cortex. 2017;27(1):117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanner AM. Depression and epilepsy: a review of multiple facets of their close relation. Neurol Clin. 2009;27(4):865–80. [DOI] [PubMed] [Google Scholar]
- 43. Martinez A, Finegersh A, Cannon DM, Dustin I, Nugent A, Herscovitch P, et al. The 5‐HT1A receptor and 5‐HT transporter in temporal lobe epilepsy. Neurology. 2013;80(16):1465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kambeitz JP, Howes OD. The serotonin transporter in depression: meta‐analysis of in vivo and post mortem findings and implications for understanding and treating depression. J Affect Disord. 2015;186:358–66. [DOI] [PubMed] [Google Scholar]
- 45. Kumar U, Medel‐Matus JS, Redwine HM, Shin D, Hensler JG, Sankar R, et al. Effects of selective serotonin and norepinephrine reuptake inhibitors on depressive‐ and impulsive‐like behaviors and on monoamine transmission in experimental temporal lobe epilepsy. Epilepsia. 2016;57(3):506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baldinger P, Kranz GS, Haeusler D, Savli M, Spies M, Philippe C, et al. Regional differences in SERT occupancy after acute and prolonged SSRI intake investigated by brain PET. Neuroimage. 2014;88:252–62. [DOI] [PubMed] [Google Scholar]
- 47. Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety‐related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–31. [DOI] [PubMed] [Google Scholar]
- 48. Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014;466(1):25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schneider I, Kugel H, Redlich R, Grotegerd D, Burger C, Burkner PC, et al. Association of Serotonin Transporter Gene AluJb methylation with major depression, amygdala responsiveness, 5‐HTTLPR/rs25531 polymorphism, and stress. Neuropsychopharmacology. 2018;43(6):1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iurescia S, Seripa D, Rinaldi M. Role of the 5‐HTTLPR and SNP promoter polymorphisms on serotonin transporter gene expression: a closer look at genetic architecture and in vitro functional studies of common and uncommon allelic variants. Mol Neurobiol. 2016;53(8):5510–26. [DOI] [PubMed] [Google Scholar]
- 51. Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–3. [DOI] [PubMed] [Google Scholar]
- 52. Murthy NV, Selvaraj S, Cowen PJ, Bhagwagar Z, Riedel WJ, Peers P, et al. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5‐HTT to [11C] DASB binding in the living human brain. Neuroimage. 2010;52(1):50–4. [DOI] [PubMed] [Google Scholar]
- 53. Sugden K, Tichopad A, Khan N, Craig IW, D'Souza UM. Genes within the serotonergic system are differentially expressed in human brain. BMC Neurosci. 2009;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1
Table 1
Appendix S1
Data Availability Statement
All data is presented in this paper and supplemental files.


