Figure 1.
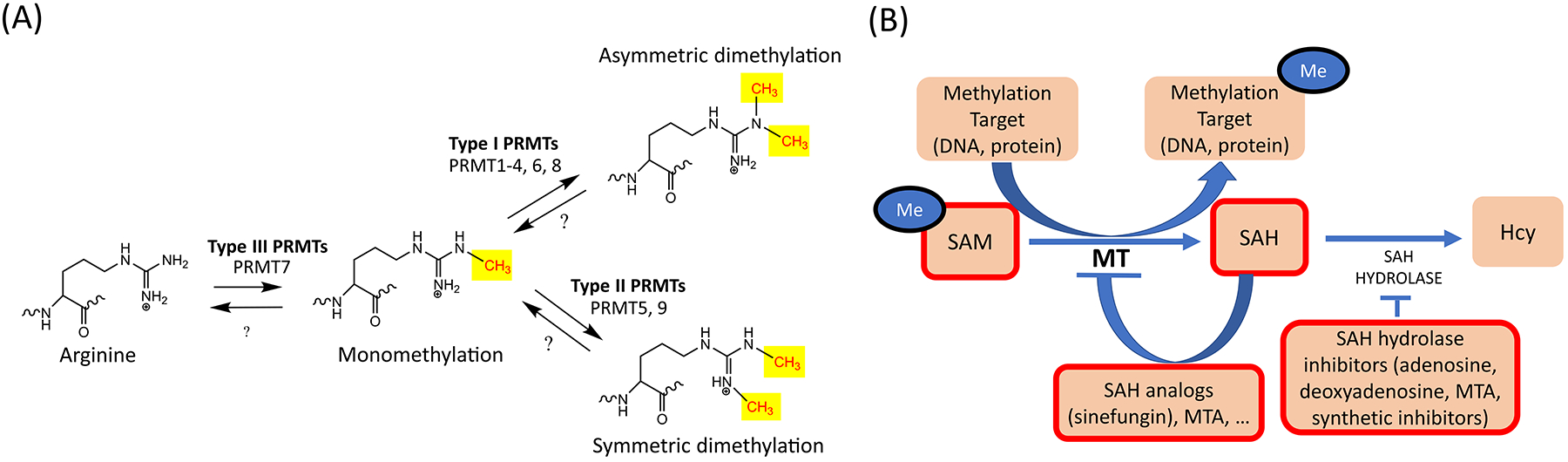
(A). Arginine methylation reactions. Arginine methylation is catalyzed by arginine methyltransferases (PRMTs), which transfer a methyl group onto the ω-nitrogen of arginine (Arg). All mammalian PRMTs modify arginine to its monomethylated state. From the monomethylated state, Type I PRMTs are able to catalyze asymmetric demethylation (ADM) of Arg [6]. Type II PRMTs are able to catalyze symmetric dimethylation (SDM) from the monomethylated state [7,8]. Type III PRMTs are solely able to catalyze monomethylation [9]. (B). S-adenosyl methionine (SAM) is the methyl donor for all methyltransferase (MT) reactions, including DNA methyltransferase (DNMT), Protein Lysine methyltransferase (PKMT), and Protein Arginine methyltransferase (PRMT) reactions. After SAM has donated its methyl group, it is converted to (S)-adenosylhomocysteine (SAH). SAH inhibits MT activity by a negative feedback loop but methylation can generally proceed if SAH is broken down by the SAH hydrolase enzyme [21]. In contrast, if SAH hydrolase is not active, SAH accumulates in the cytoplasm and inhibits SAM-dependent methylation reactions. Therefore, MT inhibitors often act by inhibiting SAH hydrolase and increasing the concentration of the natural inhibitor SAH [21]. Products with MT inhibitory activity are indicated with a thick red outline. SAM can act as an inhibitor by producing high amounts of SAH, while SAH hydrolase inhibitors do the same by inhibiting the SAH degradation [21]. Finally, SAH analogs also have general MT inhibitory activity [21].
