Abstract
Background
The major complications of “treated” Human Immunodeficiency Virus (HIV) infection are cardiovascular disease, malignancy, renal disease, liver disease, bone disease, and perhaps neurological complications, which are phenomena of the normal aging process occurring at an earlier age in the HIV-infected population. The present study is aimed to explore protein carbonyl content as a biomarker for detecting oxidative DNA damage induced ART toxicity and/or accelerated aging in HIV/AIDS patients.
Objective
To investigate the potential of carbonyl content as a biomarker for detecting oxidative Deoxyribonucleic acid (DNA) damage induced Antiretroviral Theraphy (ART) toxicity and/or accelerated aging in HIV/AIDS patients.
Methods
In this case–control study a total 600 subjects were included. All subjects were randomly selected and grouped as HIV-negative (control group) (n = 300), HIV-infected ART naive (n = 100), HIV-infected on first line ART (n = 100), and HIV-infected on second line ART (n = 100). Seronegative control subjects were age- and sex-matched with the ART naive patients and the two other groups. Carbonyl protein was determined by the method described in Levine et al. DNA damage marker 8-OH-dG was determined using 8-hydroxy-2-deoxy Guanosine StressXpress ELA Kit by StressMarq Biosciences.
Results
Protein carbonyl content levels and oxidative DNA damage were significantly higher (p < 0.05) in HIV-infected patients on second line ART and HIV-infected patients on first line ART than ART naive patients and controls. In a linear regression analysis, increased protein carbonyl content was positively associated with increased DNA damage (OR: 0.356; 95% CI: 0.287–0.426) p < 0.05.
Conclusions
Carbonyl content may has a role as a biomarker for detecting oxidative DNA damage induced ART toxicity and/or accelerated aging in HIV/AIDS patients. Larger studies are warranted to elucidate the role of carbonyl content as a biomarker for premature aging in HIV/AIDS patients.
Keywords: Carbonyl content, DNA damage, Human immunodeficiency virus (HIV) infection, 8-OH-dG, ROS
Abbreviations: ART, antiretroviral therapy; ELISA, Enzyme Linked Immunosorbent Assay; HIV, human immunodeficiency virus; 8-OHdG, 8-hydroxydeoxyguanosine; ROS, reactive oxygen species
Introduction
The AIDS epidemic continues to spread in the South-East Asia (SEA) Region. The South-East Asia is the second most affected WHO region in the world, after sub-Saharan Africa. To date, close to 40 million people throughout the world have been infected with human immunodeficiency virus (HIV). Of these, almost 6.4 million are in the SEA Region. Over 99% of cases have been reported from four countries – Thailand, India, Indonesia and Myanmar.1 More than 25 antiretroviral drugs from six therapeutic classes are now available for the management of HIV infection. Most patients who take medication achieve durable and perhaps lifelong viral suppression, so the classic AIDS related conditions are becoming less common. However, treated patients do not have completely restored health. Compared with people without HIV infection, patients with the infection who are treated with antiretrovirals have increased risk for several “non-AIDS” complications, many of which are commonly associated with aging.2
The major complications of “treated” HIV disease are cardiovascular disease, malignancy, renal disease, liver disease, bone disease, and perhaps neurological complications, which are phenomena of the normal aging process, occur at an earlier age in the HIV infected population.3
A critical research question is whether HIV is accelerating aging itself through pathways and mechanisms common to the aging process or, alternatively, HIV may simply be an additional risk factor for a wide number of chronic conditions thus accentuating the prevalence of disease at every age. This situation is further complicated by concern that toxicities of ART, not HIV itself, may contribute to aging and age-related illness. Specific biomarkers of aging are limited in HIV-infected hosts and impacted by antiretroviral therapy, and a high rate of modifiable life style confounders (e.g., smoking, substance abuse, alcohol) and co-infections (e.g., viral hepatitis) in HIV-infected participants. There is a need for validated biomarkers of aging in the context of HIV and ART.4, 5, 6, 7
To show that HIV infection is associated with accelerated normal aging, one first needs to understand what is meant by normal aging and to find a way of measuring it.
Aging, also described as organismal senescence, is defined as a state with decreased ability to respond to stress, decreased homeostatic balance, and an increased risk of developing age-related diseases.8
Oxidative stress leads to an elevated oxidation of macromolecules, such as DNA, lipids, and proteins. It was postulated that age-related accumulation of damaged, oxidized, and aggregated proteins might contribute to the aging process.8, 9
Oxidation of proteins leads to a partial unfolding and, therefore, to aggregation. Protein aggregates impair the activity of cellular proteolytic systems (proteasomes, lysosomes), resulting in further accumulation of oxidized proteins. In addition, the accumulation of highly cross linked protein aggregates leads to further oxidant formation, damage to macromolecules and finally, to apoptotic cell death. Furthermore, protein oxidation seems to play a role in the development of various age related diseases, such as neurodegenerative diseases, type 2 diabetes, cancer, and cardiovascular disease.2
Two of the most commonly measured oxidative protein modifications are protein carbonyls and 3-NT, which are used to assess the cellular status of protein oxidation. Protein carbonyls are generated due to the oxidation of proline, arginine, lysine, threonine, and other amino-acid residues and due to the oxidation of the protein backbone. Moreover, they might be the result of secondary reactions of amino acids (cysteine, histidine, and lysine) with reactive carbonyl compounds (ketones, aldehydes), emerged in lipid peroxidation or glycation/glycoxidation reactions.9 One way to measure protein oxidation is to determine levels of protein carbonyls.10
Under normal physiological conditions in all aerobic organisms, there is a balance maintained between endogenous oxidants and numerous enzymatic and non-enzymatic antioxidant defences. When an imbalance occurs, oxidants produce extensive oxidative damage to DNA, which, in turn, contributes to aging, malignant tumors, and other degenerative diseases.11, 12 Oxidative injury is integral to HIV/AIDS as a potent inducer of viral activation, viral replication, and DNA damage in infected cells.13, 14
Although many damaged DNA lesions have been identified, we have chosen 8-hydroxy-2-deoxyguanosine (8-OHdG) as our biomarker of oxidative damage. The importance of this lesion stems from the fact that it is both abundant in DNA and it is mutagenic. Current evidence suggests that 8-OHdG lesions present in DNA during cellular replication results in somatic mutation, the driving force behind carcinogenesis.13, 15 Determination and analysis of 8-OHdG can be performed in animal organs and in human sample (urine, human organs, leukocyte DNA) as a biomarker of oxidative stress, aging, and carcinogenesis.16, 17 Many studies in the past 20 years and improvements in the quantitative estimation of 8-OHdG by various analytical techniques in blood cells or in urine have established it as a very important biomarker not only for carcinogenesis but also for aging and degenerative diseases.18, 19, 20, 21
The search of suitable biomarkers for aging in HIV/AIDS patients is ongoing. There is need of biomarker for premature aging that could alert the clinician to further investigate non AIDS related diseases in HIV/AIDS patients. Such a tool should be inexpensive and facilities for its determination easily available.
In this study we determined protein oxidation marker carbonyl content and correlated it with DNA damage marker 8-OHdG in HIV-infected patients on ART, ART naïve, and HIV-negative controls.
Methods
Subject selection
A case–control study was carried out on HIV-1 infected patients at the outpatient infectious disease unit (OPD) and ART centre of the Sir J J Hospital & Grant Government Medical College, Mumbai over a period of one year, from February 2014 to March 2015. We have selected 300 subjects from OPD after evaluation of their medical records (negative serial ELISA/Western blot test for HIV before three months of sample collection) as HIV-negative controls and 300 HIV-infected subjects detected by serial ELISA/Western blot method from ART centre. All subjects were randomly selected and grouped as HIV-negative (control group) (n = 300), HIV-infected ART naive (n = 100), HIV-infected on first line ART (n = 100) and HIV-infected on second line ART (n = 100). Seronegative control subjects were age- and sex-matched with the ART naive patients and the two other groups. We used power analysis method for sample selection at 5% significance level for 95% confidence interval.
We do not have data regarding baseline viral load, CD4 nadir, and viral suppression after starting ART for the study population, as these are not included in routine tests for HIV/AIDS patients at ART centre of the Sir J J Hospital &Grant Government Medical College, Mumbai. As this was a self-funded study we could not perform these tests due to limited funds.
Ethical approval
The study protocol was approved by the institutional ethics committee (No. IEC/Pharm/902/2013) and National AIDS Control Organization Delhi, India (T-11020/67/2011-NACO).
Inclusion criteria
All participants were 20 years of age or older HIV-infected patients detected by serial ELISA/Western blot method and were included in this study after giving their informed consent. We have selected 300 subjects from OPD after evaluation of their medical records (negative serial ELISA/Western blot test for HIV before three months of sample collection) as HIV-negative controls (n = 300).
Exclusion criteria
Exclusion criteria included pregnant women, patients with chronic diseases like hepatitis, diabetes, renal impairment, cardiovascular co-morbidities, neurological psychiatric disorders, various malignancies, as well as heavy smokers, alcoholics, tobacco-chewers, and HIV-infected patients with withdrawal of combination ART. The demographic details were collected from each patient and entered into the pro-forma. Subsequent to this, a detailed history was taken.
Sample collection
Venous blood samples were collected in plain and lithium heparin vacutainers as an anticoagulant. Blood was centrifuged (4000 g, 10 min, 4 °C) to separate the plasma. The collected plasma was stored at −70 °C with aseptic precautions. Plain blood samples 2 h after collection were centrifuged at 3000 rpm for 5 min; serum was separated and collected in sterile tubes.
Different treatment regimens as per NACO guidelines
The list of antiretroviral therapy administered to Indian HIV-infected patients was as follows:
| First line therapy | Second line therapy |
|---|---|
| Tenofovir | Tenofovir |
| Lamivudine | Lamivudine |
| Efavirenz | Ritonavir |
| Atazanavir |
Biochemical methods
Determination of protein carbonyl content
Protein carbonyl proteins were assayed by 2,4-dinitrophenylhydrazine reaction.22 The assay is based on the spectrophotometric detection of the reaction between 2,4-dinitrophenyl hydrazine (DNPH) with protein carbonyl to form protein hydrazone. Carbonyl content was determined as nmol mg−1 protein. The total protein content was measured using colorimetric kit based on biuret method.
Determination of DNA damage marker 8-hydroxy-2-deoxyguanosine (8-OHdG)
Plasma of all the cases and control samples was used for the measurement of 8-OHdG levels using competitive in vitro Enzyme-Linked Immunosorbent Assay (ELISA) kit obtained from StressXpress ELA Kit by StressMarq Biosciences. 8-OHdG measurements were performed using microtiter ELISA plate pre-coated with anti-mouse IgG. 50 μL sample, 50 μL 8-OHdG AChE (acetylcholinesterase) tracer and 50 μL 8-OHdG monoclonal antibody were added to each well and incubated at 4 °C for 18 h. After the wells were washed five times, 200 μL Ellman's reagent was added to each well. The wells were incubated at room temperature in the dark for 100 min. The absorbance was read at wavelength of 420 nm. ELISA assay displays IC50 (50% B/B0) and IC80 (80% B/B0) values of approximately 100 and 30 pg mL−1, respectively.
Statistical methods
Data were expressed as the mean ± SD. Student's t test was performed to assess differences between two means. Statistical analysis was performed using EPI-INFO 7 statistical software for medical research studies. Differences in means between groups were tested using independent-sample t-tests. p < 0.05 was considered as statistically significant. Mann–Whitney test for non parametric continuous variables, Chi-square or Fisher's exact test for categorical variables were used to test for statistical significance. We used linear regression method for correlation of protein carbonyl content and DNA damage marker 8-OH-Dg, and we used logistic regression method for comparison between two age groups and ART second-line, ART first-line, and ART naïve.
Results
A total of 600 (300 HIV-infected and 300 HIV negative) subjects were included in this study. All the subjects were divided in two age groups (20–40 yrs) and (40–60 yrs). As seen in Table 1, the mean carbonyl content levels were significantly higher than that of the control group for the ART-naïve (p < 0.001) group, those on first-line ART (p < 0.001) and on second-line ART (p < 0.001) in both age groups. DNA damage marker levels were also significantly higher than that of the control group for the ART naïve (p = 0.001), those on first-line ART (p < 0.001) and on second-line ART (p < 0.001) in both age groups. Carbonyl content and DNA damage marker levels correlated positively with each other for the total patient sample (OR: 0.356; 95% CI: 0.287–0.426) [p < 0.001]. These results supported the notion of carbonyl content as the better marker of inflammatory activity and are in agreement with the concept of ART toxicity as a condition with increased inflammatory activity with DNA damage.
Table 1.
Patients characteristics, CD4 count, plasma DNA damage marker 8-OHdG, and serum carbonyl content.
| Group | Subgroup | Male/female | Age (mean) | CD4 (mean) | Time on anti-retroviral therapy (mean) | 8-OHdG | Carbonyl content |
|---|---|---|---|---|---|---|---|
| count | years | cells/μL | months | ng/mL | nmol/mg of protein | ||
| HIV-negative controls | 20–40 yrs | 87/63 | 34.8 ± 5.1 | NA | NA | 1.07 ± 0.25 | 0.64 ± 0.08 |
| 40–60 yrs | 92/58 | 47.3 ± 5.4 | NA | NA | 1.30 ± 0.32 | 0.66 ± 0.10 | |
| HIV-infected ART naive | 20–40 yrs | 28/22 | 31.6 ± 4.2b | 624 ± 202b | NA | 1.81 ± 0.66a | 0.76 ± 0.13a |
| 40–60 yrs | 33/17 | 45.2 ± 6.1b | 602 ± 134b | NA | 1.95 ± 0.56a | 0.77 ± 0.18a | |
| HIV-infected on first line ART | 20–40 yrs | 30/20 | 34.3 ± 5.2b | 502 ± 233b | 63.6a | 2.50 ± 0.91a | 1.06 ± 0.31a |
| 40–60 yrs | 41/9 | 48.3 ± 4.8b | 465 ± 165b | 65.7a | 3.13 ± 0.92a | 1.08 ± 0.37a | |
| HIV-infected on second line ART | 20–40 yrs | 36/14 | 35.6 ± 4.3b | 400 ± 144b | 74.3a | 2.79 ± 0.95a | 1.20 ± 0.36a |
| 40–60 yrs | 43/7 | 47.5 ± 5.3b | 376 ± 183b | 76.8a | 3.27 ± 0.99a | 1.22 ± 0.36a | |
Values are mean ± SEM of subjects. The data were analyzed by one-way ANOVA.
p < 0.01 significant when compared to control.
p < 0.05 significant when compared to control.
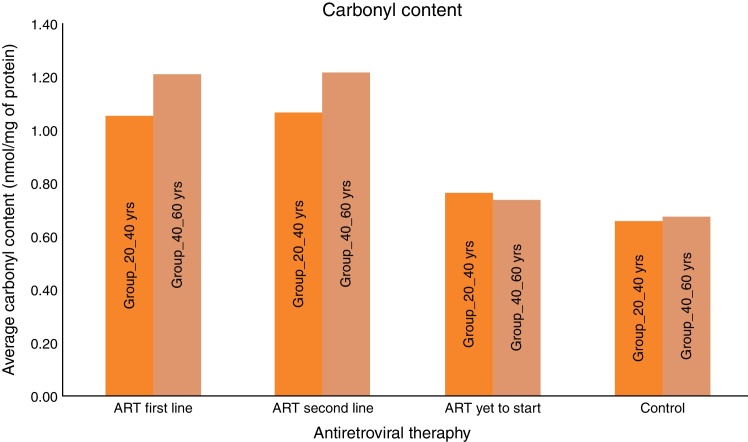
Carbonyl content was significantly higher in HIV-infected patients on ART than HIV-infected ART naïve subjects and HIV negative controls. The mean carbonyl content for HIV-infected ART naive patients in age group 20–40 yrs was 0.76 nmol/mg of protein and in age group 40–60 yrs was 0.74 nmol/mg of protein. The mean carbonyl content for HIV-infected in both age groups, 20–40 yrs and 40–60 yrs on ART (first line) were 1.06 and 1.21 nmol/mg of protein and 1.06 and 1.22 nmol/mg of protein for those on second line.
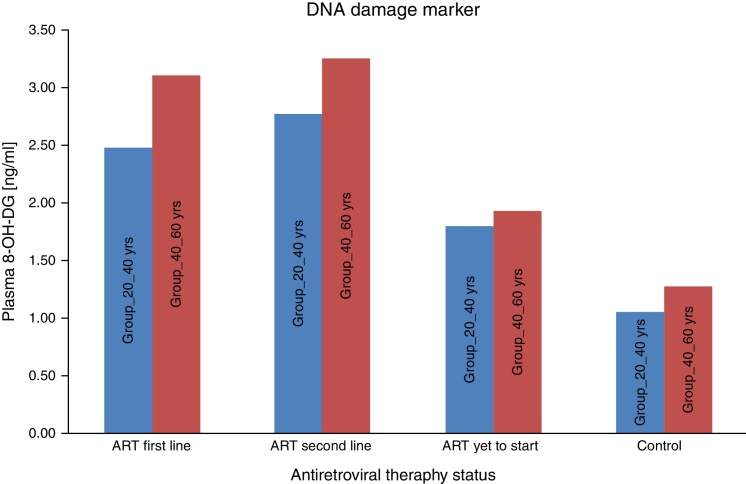
We consider the normal value for carbonyl content is up to 0.75 nmol/mg of protein and more than 0.75 nmol/mg of protein is considered as abnormal value of carbonyl content.22 Carbonyl content was abnormal in 14 (28%) ART naive HIV-infected patients in the age group 20–40 yrs and in 17 (34%) in the age group 40–60 yrs. The carbonyl content was significantly more abnormal in HIV-infected patients in both age groups (20–40 yrs and 40–60 yrs) on ART: 31 (62%) on first line and 33 (66%) on second line, and 35 (70%) and 38 (76%), respectively. These results show that carbonyl content was increased in HIV-infected patients on ART. In a logistic regression analysis carbonyl content was significantly different between the age groups of 20–40 yrs and 40–60 yrs (OR: 0.609; 95% CI: 0.4502–0.8258 – p < 0.001), between second-line and first-line ART (OR: 0.7959; 95% CI: 0.5183–1.222 – p < 0.296), and between naïve patients and those on first-line (OR: 5.717; 95% CI: 3.762–8.672 – p < 0.001). The mean 8-OH-DG for HIV-infected patients was higher than that for HIV negative subjects. The mean 8-OH-DG for HIV-infected ART naive patients in age group 20–40 yrs was 1.81 ng/mL and in age group 40–60 yrs was 1.95 ng/mL. The mean 8-OH-DG for HIV-infected in both age groups (20–40 yrs and 40–60 yrs) on ART (first line) were 2.50 ng/mL and 3.13 ng/mL, and (second line) 2.79 ng/mL and 3.27 ng/mL, respectively. ART accelerates DNA damage in HIV-infected patients. These results show increased DNA damage in HIV-infected patients on ART. In this study we used linear regression method to correlate protein carbonyl content with DNA damage. We observed that increased protein carbonyl content levels were positively associated with increased DNA damage (OR: 0.356; 95% CI: 0.287–0.426 – p < 0.001). Increased mean carbonyl content values were observed in HIV-infected patients on first line and second line ART of both age groups (20–40 yrs and 40–60 yrs) than ART naive and negative controls; similar pattern was observed with DNA damage marker 8-OH-DG in HIV-infected patients on first line and second line ART of both age groups (20–40 yrs and 40–60 yrs), as shown in Table 1.
Discussion
Infection with the human immunodeficiency virus-1 (HIV) is associated with clinical symptoms of accelerated aging, as evidenced by increased incidence and diversity of age-related illnesses at relatively young ages. Cardiovascular disease, diabetes, and several other conditions are more prevalent at all ages in those with HIV, suggesting there is an extra “hit” by HIV and/or ART – that is, accentuated aging.4, 5, 6, 7 There is considerable evidence suggesting that oxidative stress plays a critical role in both in vitro senescence and in vivo aging.23 Telomeres cap the ends of chromosomes and consist of hexameric TTAGGG repeats and the protective ‘shelterin’ protein complex. Telomerase inhibition has been considered a possible mechanism by which ART in HIV/AIDS causes accelerated aging.24
A very important marker of oxidative stress is protein carbonylation, measured through estimating protein carbonyl groups content in serum. Measuring protein carbonyl content is advantageous over other biomarkers of oxidative stress due to their early formation and detectable stability arising from protein side chains (Pro, Arg, Lys, and Thr).25 Protein carbonylation is a type of protein oxidation that can be promoted by reactive oxygen species. It usually refers to a process that forms reactive ketones or aldehydes that can be reacted by 2,4-dinitrophenylhydrazine (DNPH) to form hydrazones.26 Oxidative modification of proteins is known to affect protein function. Protein carbonyls and protein nitrotyrosine are widely used and chemically stable biomarkers of protein oxidation. The protein carbonyls (PC) group are formed by either direct oxidation of certain amino acid residues, particularly lysine, arginine, threonine, proline, and histidine or secondarily reaction with product of lipid peroxidation (e.g., HNE) or glycoxidation reaction with lysine group.27 Protein carbonyls are better studied than protein nitration. The concentration of PC was stable, yielded quantitative results, and appeared to reflect disease endpoints in a biologically significant way. Carbonylated proteins are relatively difficult to induce compared with the SH oxidation of cysteine and methionine, and elevated levels of carbonylation are thought to be a sign not only of oxidative stress, but also of disease-derived protein dysfunction. Therefore, carbonylated proteins can be used as oxidative stress biomarkers according to their special properties including irreversibility, unrepairability, stability in physical condition and induction of protein aggregation. Proteins are major bio-molecules that drive cellular activities. Growing evidence suggests that onco-proteins, tumor suppressor proteins and oxidative stress-related proteins play significant roles in carcinogenesis.27 Aging is accompanied by accumulation of damaged DNA, misfolded proteins, and oxidized proteins. A multitude of data indicate that protein oxidation, accumulation of oxidized proteins, and protein aggregation as well as the impairment of the proteosomal system play a major role in the aging process and in the development of some, if not all, age-related diseases.28
Free radicals may also bring about the oxidative damages of DNA that are manifested by the development of various complications. The oxidatively induced DNA damage typically associated with ROS are apurinic/apyrimidinic (AP) DNA sites, oxidized purines and pyrimidines, single strand and double strand DNA breaks. The accumulation of oxidative DNA damage may be linked with age-associated neurodegenerative disorders Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis. ROS generate 8-oxodG, which can be used as an indicator of oxidative DNA damage in relation to oxidative stress-driven diseases. Kryston et al. reviewed the role of oxidative stress and DNA damage in human malignancies, and suggested the idea of using several oxidatively generated DNA lesions like 8-oxo-7,8-dihydro-2′-deoxyguanine (8-oxodG), also known as 8-hydroxydG (8-OHdG), thymine glycol, AP sites and oxidatively generated clustered DNA lesions (OCDLs) as novel biomarkers of oxidative stress.16 The most commonly used marker of oxidatively modified DNA is 8-hydroxy-2′-deoxyguanosine (8-OHdG), a product of oxidatively modified DNA base guanine. DNA can be oxidized to produce many oxidative products; however, oxidation of the C-8 of guanine is one of the most common oxidative events and results in a mutagenic lesion that produces predominantly G-to-T transversion mutations. 8-OHdG was found to be increased in a normal human LEC culture after induced oxidative stress.17 Biological materials most often used to measure levels of 8-OHdG include, serum, plasma, urine and tissues. In recent years 8-hydroxydeoxyguanosine (8-OHdG) has emerged as a marker of oxidative damage in the patho-physiological processes of cancer.18, 19 Levels of 8-OHdG in various biological samples has been correlated with disease activity, thus 8-OHdG is a useful marker for study of DNA damage caused by free radicals. There is ample circumstantial evidence that oxidative stress leads to the accumulation of DNA damage during aging.20 These data suggest that carbonyl content and 8-OHdG levels are potentially useful biomarkers of aging in HIV/AIDS patients. It will be important to account for differential exposure to risk factors (e.g., smoking, alcohol, non-HIV sexually transmitted diseases) between HIV-infected and HIV-uninfected populations that are likely to result in residual confounding when assessing associations of HIV with increased risk of age-related illness. Therefore, we have chosen the subject population and control population with similar economical background and similar confounders (smoking and alcohol).
All experiments described in this study were performed blindly with respect to subject status (ART naïve, ART first-line, ART second-line HIV patients, and control subjects). In the present study the value of carbonyl content as general marker of the inflammatory status was examined by comparing carbonyl content levels to that of other recognized biomarker of inflammation, i.e., DNA damage marker 8-OHdG. As seen in Fig. 1, the mean carbonyl content levels were significantly higher than that of the control group for the ART-naïve patients (p < 0.001), those on first-line ART (p < 0.001), and those on second-line ART (p < 0.001). 8-OHdG levels were also significantly higher among ART naïve patients (p = 0.001), those on first-line ART (p < 0.001), and those on second-line ART (p < 0.001) than those of the control group (Fig. 2).
Fig. 1.
Average carbonyl content among subjects.
Fig. 2.
Average DNA damage marker among subjects.
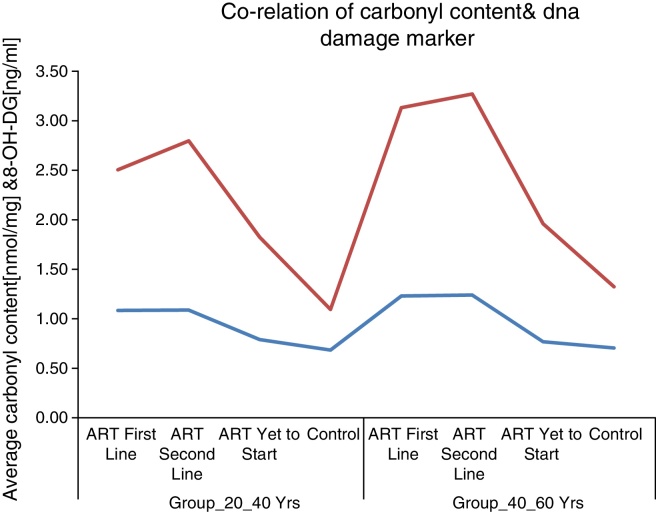
These results supported the notion of carbonyl content as a better marker of inflammatory activity and are in line with the concept of advanced HIV/AIDS as a condition with increased inflammatory activity. Protein carbonyl content and 8-OHdG levels are significantly increased after ART in HIV/AIDS patients of both age groups (Fig. 1, Fig. 2). Significant increase in protein carbonyl content and 8-OHdG levels were observed in HIV-infected patients in the age group 40–60 years when compared to those in the age group 20–40 years. In fact, protein carbonyl content levels show a strong and significant correlation with DNA damage. Thus we have observed a close association between carbonyl content and DNA damage in HIV/AIDS with ART (Fig. 3). This suggests that the accumulation of carbonyl content may be an early indication of increased DNA damage marker 8-OHdG levels which may indicates accelerated aging in HIV/AIDS patients on ART.
Fig. 3.
Co-relation of carbonyl content & DNA damage marker.
Inflammation and activation of coagulation pathways are central to the pathophysiology leading to morbidity and mortality in HIV-infected patients, as demonstrated by data from the Strategies for Management of Antiretroviral Therapy study, which showed that IL-6 and D-dimer were strongly related to non-AIDS – defining comorbidities and all-cause mortality in patients on ART. In HIV infection, the heightened inflammation and immune dysfunction related to accelerated aging has stimulated assessment of several relevant biomarkers including C-reactive protein, IL-6, and D-dimer and their associations with age-related morbidity and HIV infection.4 A weakness of the present study was that those biomarkers could not included.
We do not yet know if antioxidant therapy is useful to prevent premature aging in HIV/AIDS patients. This study demonstrated the extent of oxidative protein damage and its association with DNA damage. Hence determination of protein carbonyl content can serve as a simple biomarker for monitoring the magnitude of oxidative DNA damage induced ART toxicity and/or accelerated aging in HIV/AIDS patients. Further studies are needed to clarify this issue.
Conclusion
This study in HIV/AIDS patients showed carbonyl content as a better indicator of the premature aging due to effect of ART. It is clear that therapeutic side-effects and effects of HIV-1 infection together must be considered in the pathogenesis of aging in the HIV/AIDS population, as antiretroviral therapy is linked directly to the infection. There is an abundance of evidence implicating ROS as one source of DNA damage associated with aging, cancer, stress signaling, and other conditions. In the present study we observed that carbonyl content is positively correlated to DNA damage marker 8-OHdG levels in HIV/AIDS patients. Overall, our results demonstrate that the carbonyl content may have a role as a biomarker for detecting oxidative DNA damage induced ART toxicity and/or accelerated aging in HIV/AIDS patients. Determination of carbonyl content is a very simple and cost-effective method hence we suggest that carbonyl content may be used as a biomarker for determination of ART toxicity and/or premature aging in HIV/AIDS patients. In future, management of premature aging and DNA damage in HIV/AIDS patients should be prime concern for health care physicians.
Authors contribution
VK carried out the sample and data collection. VK carried out all experimental work, data analysis and draft the manuscript. VP assisted VK in experimental work and draft designing. VP helped in subject selection and sample selection. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Vajpayee M., Mohan T. Current practices in laboratory monitoring of HIV infection. Indian J Med Res. 2011;134:801–822. doi: 10.4103/0971-5916.92627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wendelken L., Valcour V. Impact of HIV and aging on neuropsychological function. J Neurovirol. 2012;18:256–263. doi: 10.1007/s13365-012-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks S.G. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathai S., Bajillan H., Landay A.L., High K.P. Is HIV a model of accelerated or accentuated aging? J Gerontol A: Biol Sci Med Sci. 2014;69:833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvath S., Levine A.J. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212:1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin S., Cremer J., Roll P. HIV-1 infection and first line ART induced differential responses in mitochondria from blood lymphocytes and monocytes: the ANRS EP45 “aging” study. PLoS ONE. 2012;7:e41129. doi: 10.1371/journal.pone.0041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rickabaugh T.M., Baxter R.M., Sehl M. Acceleration of age-associated methylation patterns in HIV-1-infected adults. PLoS ONE. 2015;10:e0119201. doi: 10.1371/journal.pone.0119201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Höhn A., Konig J., Grune T. Protein oxidation in aging and the removal of oxidized proteins. J Proteomics. 2013;92:132–159. doi: 10.1016/j.jprot.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Stadtman E.R., Berlett B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- 10.Dalle-Donne I., Rossi R., Giustarini D. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 11.Smith C.D., Carney J.M., Starke-Reed P.E. Excess brain protein oxidation and enzyme dysfunction in normal Aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starke-Reed P.E., Oliver C.N. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989;275:559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- 13.Stadtman E.R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 14.Kryston T.B., Georgiev A.B., Pissis P. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Aukrust P., Luna L., Ueland T., Johansen Impaired base excision repair and accumulation of oxidative base lesions in CD4+ T cells of HIV-infected patients. Blood. 2005;105:4730–4735. doi: 10.1182/blood-2004-11-4272. [DOI] [PubMed] [Google Scholar]
- 16.Valavanidis A., Vlachogianni T., Fiotakis C. 8-Hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 17.Olinski, Rozalski, Gackowski Urinary measurement of 8-oxodg, 8-oxogua, and 5HMUra: a non invasive assessment of oxidative damage to DNA. Antioxid Redox Signal. 2006;8:1011–1019. doi: 10.1089/ars.2006.8.1011. [DOI] [PubMed] [Google Scholar]
- 18.Yermilov V., Rubio, Becchi, Friesen, Pignatelli Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16:2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez, Song, Crouse In vivo bypass of 8-oxodG. PLoS Genet. 2013;9:e1003682. doi: 10.1371/journal.pgen.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melis, van Steeg, Luijten M. Oxidative DNA damage and nucleotide excision repair. Antioxid Redox Signal. 2013;18:2409–2419. doi: 10.1089/ars.2012.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiraku, Kawanishi, Ichinose The role of iNOS-mediated DNA damage in infection- and asbestos-induced carcinogenesis. Ann N Y Acad Sci. 2010;1203:15–22. doi: 10.1111/j.1749-6632.2010.05602.x. [DOI] [PubMed] [Google Scholar]
- 22.Levine R.L., Garland D., Oliver C.N., et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 23.Torres R.A., Lewis W. Aging and HIV/AIDS: pathogenetic role of therapeutic side effects. Lab Invest. 2014;94:120–128. doi: 10.1038/labinvest.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey G., Shihabudeen M.S., David H.P. Association between hyperleptinemia and oxidative stress in obese diabetic subjects. J Diabetes Metab Disord. 2015;14:24. doi: 10.1186/s40200-015-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki Y.J., Carini M., Butterfield A.D. Protein carbonylation. Antioxid Redox Signal. 2010;12:323–325. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeg S., Grune T. Protein oxidation in aging: does it play a role in aging progression? Antioxid Redox Signal. 2015;23:239–255. doi: 10.1089/ars.2014.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thanan R., Oikawa S., Hiraku Y. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2015;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedelnikovaa O.A., Redona C.E. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]