Abstract
Human T cell lymphotropic virus type 1 (HTLV-1) was the first retrovirus discovered in humans and is endemic in several parts of the world. Because of risk behaviors, mainly sexual, men who have sex with men (MSM) are at high risk of acquiring HTLV-1 infection. A cross-sectional study was performed to estimate the prevalence of HTLV-1 infection, to characterize genetically HTLV-1 sequences and to identify risk behaviors associated with this infection among MSM in Central Brazil. A total of 430 MSM were enrolled in this study and three were shown to be HTLV-1 infected, prevalence of 0.7% (95% confidence interval: 0.4–0.9). Phylogenetic analysis showed that all HTLV-1 positive samples belonged to Cosmopolitan subtype Transcontinental subgroup A. Although the prevalence rate of HTLV-1 infection found in this study was similar to that observed among Brazilian blood donors, additional HTLV-1 preventive interventions need to be further implemented because this population is engaged in high-risk sexual behavior.
Keywords: HTLV-1, Men who have sex with men, Sexual risk behavior, Molecular epidemiology, Brazil
Introduction
The human T cell lymphotropic virus type 1 (HTLV-1) is a retrovirus belonging to Retroviridae family, Orthoretrovirinae subfamily and Deltaretrovirus genus. Two main diseases are associated with the virus, adult T-cell leukemia and the neurological disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), besides inflammatory syndromes.1 The most important routes of HTLV-1 transmission were found to be vertically from mother to child (breastfeeding), sexual intercourse, and blood contact.1, 2
Worldwide, the number of carriers of HTLV-1 infection is estimated to be at least five to ten million infected individuals. Southwestern Japan, Caribbean islands, the Middle East, Australo-Melanesia, Sub-Saharan Africa, and South America are considered endemic areas of this infection. Based on analyses of long terminal repeat (LTR) region of several HTLV-1 isolates worldwide, seven different viral subtypes were defined (a–g). The most widely circulating HTLV-1 strain around the world belongs to the Cosmopolitan (a) subtype.3
Besides geographical location, the prevalence of HTLV-1/2 also varies depending on the population groups in Brazil. HTLV-I prevalence rate among blood donors was 0.48% in a study conducted in different blood centers across the country.4 Furthermore, high-risk groups include sex workers, injection drug users, Japanese immigrants, and men who have sex with men (MSM).2, 5, 6
Due to risk behaviors, such as greater number of lifetime male partners and unprotected sexual intercourse, MSM are considered highly vulnerable for acquiring sexually transmitted infections (STIs).7, 8 There is a paucity of information on the prevalence of HTLV-1/2 among MSM. Studies in this population are more commonly related to HIV infection.9, 10
Considering the relevance of HTLV-1 infection and lack of data related to infection among MSM, the aim of the present study was to assess HTLV-1 infection prevalence and to identify the circulating HTLV-1 types and subtypes in MSM from Central Brazil.
Materials and methods
Study population
This cross-sectional study was performed in Campo Grande city (786,797 inhabitants), the State capital of Mato Grosso do Sul, Central Brazil, from November 2011 to September 2013. A convenience sampling of MSM was selected primarily from entities related to this group such as the Mato Grosso do Sul State Association of Travestites and Transsexuals (ATMS-MS) and Reference Center of Human Rights in the Prevention and Combat of Homophobia (CentrHo). In addition, public (square, parks, streets, gay pride parades, etc.) and private (saunas, nightclubs, brothels, etc.) locations were also included as recruitment sites of participants. Males who self-reported having had sexual intercourse with men within 12 months prior to the study and older than 18 years of age were eligible to participate. The minimum sample size calculated was 402 participants, based on the mean prevalence of 2% for anti-HTLV-1/2 among MSM,6, 7 a significance level of 95% (α < 0.05), 1.5% of accuracy and 20% non-respondent.
After reading and signing the informed consent form, all consenting participants were interviewed by trained health professional using a questionnaire to obtain data about study subjects and analyze its association with HTLV infection. The variables tested included socio-demographic data (such as age, marital status, educational level, family income); sexual behaviors (such as weekly number of sexual partners, engagement in diverse sexual practices, condom use in last sexual intercourse, history of sexual coercion); being sex worker (history of having ever exchanged money or goods for sex, age of sex work initiation, condom use during sex with clients and/or non-paying partners, and place of sex work); use of drugs (injection and non-injection drugs); alcohol consumption; having tattoo and/or piercing, and blood transfusion history.
After the interview, blood was collected from all subjects and serum samples were tested for HTLV-1/2-specific antibodies by enzyme-linked immunosorbent assay (ELISA) using commercial kit (Gold ELISA HTLV-I/II, REM Ind.Com., Brazil). Positive samples in the ELISA screening test were confirmed by Western blot (HTLV BLOT 2.4, MP Diagnostics, Singapore). All HTLV-1 positive samples were also tested by ELISA for HIV-1/2 infection (Murex, Kyalami, South Africa).
In exchange for participation, the subjects received condoms, lubricants and risk reduction counseling on sexually transmitted infections. Those participants found to be infected with HTLV-1 were subsequently referred to an infectious disease clinic for further clinical and laboratory assessment.
The protocol used in the present study was approved by the Ethics Committee on Human Research of the Federal University of Mato Grosso do Sul, under protocol number 180.909 CAAE 06886712.3.0000.0021.
Statistical analysis
Data were entered and analyzed using EPI-INFO 3.5.1 (Centers for Disease Control and Prevention, Atlanta, GA, USA) statistical software package and SPSS Statistics Data Editor (Statistical Package for Social Science, Chicago, IL, USA), respectively. Prevalence was calculated with 95% confidence interval (CI). Chi-square test and Fisher's exact test were used to compare categorical variables. A p-value <0.05 was defined as statistically significant.
HTLV-1 molecular characterization
Proviral DNA was obtained from whole-blood samples of the HTLV-1 infected individuals using the DNA Genomic Purification Kit (Wizard® Genomic, Promega, Madison, WI, USA), according to manufacturer's instructions. The amplification of tax and long terminal repeat (5′ LTR) regions of the HTLV-1 proviral DNA by nested-PCR was carried out as described previously.11, 12 PCR products of tax (468 bp) and 5′ LTR (485 bp) region were purified using Illustra™ GFX™ PCR DNA and Gel Band Purification Kit (GE Healthcare), according to manufacturer's instructions. The amplicons were sequenced using BigDye Terminator Cycle Sequencing Ready Reaction Kit and ABI 1373 (Applied Biosystems, Foster City, CA, USA).
Phylogenetic analysis
The 5′ LTR sequences were aligned using BioEdit v7.2.3 (Department of Microbiology, North Carolina State University, NC, USA) and edited by use of Clustal W program. MEGA software (version 6) and Tamura-Nei model were used to construct a Maximum Likelihood (ML) tree, which was evaluated by analyzing 1000 bootstrap replicates. GenBank accession numbers of the HTLV-1 sequences newly reported in this study are as follows: HSH13 (KM023762) and HSH76 (KM023763).
Results
Of the 530 MSM invited to participate of the study, 430 consented to answer the questionnaire and to have a blood sample collected. Median age of participants was 23 years, ranging from 18 to 70 years. Having less than 10 years of formal education was reported by 24.9% and low family income by 21.6% of subjects. Majority were single (87.4%) and from Campo Grande city (77.7%). Alcohol consumption was reported by most of the subjects (81.6%). Use of one or more types of illicit non-injection drugs was reported by 48.8% of participants; among those reported, marijuana was the most commonly used drug (77.1%). Only five subjects of the study (1.2%) reported illicit injection drug use. Presence of tattoos and/or body piercings was reported by 52.3% of subjects. History of blood transfusion was informed by 30 MSM (7.0%), of whom 18 received a blood transfusion before 1993.
Out of the total MSM surveyed, 37.0% were sex workers and public places were the most frequent location of sex work (66.7%). Mean age of sexual initiation and sex work debut were 14.8 years (standard deviation [SD] = ± 3.044) and 16.7 years (SD = ± 3.289), respectively. Majority of individuals reported having had more than seven clients in the week before the interview, and the frequency of irregular condom use with the last client was 32.1%. On the other hand, about half of the participants used condom regularly with their steady partner. Part of MSM (35.8%) reported having engaged in a variety of sexual practices, including masochism (35.9%), rimming (53.6%), group sex (10.5%), water sports (28.1%), shared sex toys, fisting and others (20.3%). Sixteen percent of the MSM group suffered sexual coercion. Significant differences in relation to commercial sex worker and multiple sexual partners (more than 10) was found between HTLV-1-infected and non-infected MSM.
The prevalence of HTLV-1 infection among the study population was 0.7% (3/430; 95% CI: 0.4–0.9). All samples confirmed anti-HTLV-1-reactivity with complete profiles in Western Blot test. No sample had positive reaction for anti-HTLV-2 antibodies. Table 1 shows risk behavior characteristics of all HTLV-1 positive MSM. None of HTLV-1-infected subjects were HIV-1/2-infected.
Table 1.
Sociodemographic and risk behavior characteristics of the three anti-HTLV-1 infected MSM, Campo Grande – MS, 2012–2013.
| Characteristics | ID-13 | ID-28 | ID-76 |
|---|---|---|---|
| Age(years) | 61 | 19 | 24 |
| Education(years) | 5 | 12 | 9 |
| Marital status | Single | Single | Single |
| Blood transfusion before 1993 | Yes | No | No |
| Frequency of drinking alcohol in the last 4 weeks | Drinks at least once a month | Drinks at least once a week | Drinks at least once a week |
| Ever used an illicit drug | Yes | No | No |
| Sex worker | Yes | Yes | Yes |
| Place of sex work | Residence | Street | Street |
| Age at first received money for sex(years) | 16 | 15 | 18 |
| Number of sexual partners in the last week | 2 | 20 | 15 |
| Condom use with clients | Always | Almost always | Always |
| Condom use with steady partner | Always | Almost always | Always |
ID, identification number of the MSM serological sample.
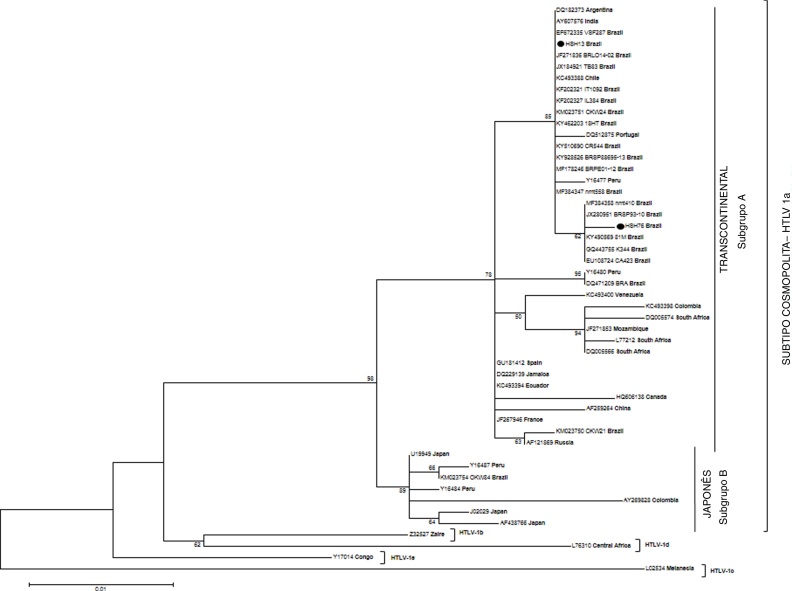
Phylogenetic analysis of 5′ LTR region of HTLV-1 sequences was possible in samples of two infected MSM (HSH13 and HSH76). Their sequences, clustered in Transcontinental subgroup (A) of Cosmopolitan HTLV-1a subtype, were closely related to other Latin American strains, mainly from Brazil, and two more strains from Portugal and India (Fig. 1). In addition, only one of the three samples (HSH28) could not be amplified in 5′ LTR region of HTLV genome, only in tax region. After sequencing, this isolate was subjected to BLAST (Basic Local Alignment Search Tool, http://blast.ncbi.nlm.nih.gov/Blast.cgi) to characterize HTLV-1 viral subtype. This procedure detected the same subtype identified previously in another sequences of the study, the Cosmopolitan HTLV-1a subtype Transcontinental subgroup A (data not shown).
Fig. 1.
Phylogenetic tree constructed using isolates based on long terminal repeat (5′ LTR) region of 485 bp of HTLV-1, including 48 sequences available in GenBank and two sequences described in this study (HSH-13 and HSH-76) by Maximum Likelihood method. The numbers on the tree denote bootstrap value (1000 replicates). Only bootstrap values of 50% or more are shown.
Discussion
This is the first cross-sectional survey of a MSM convenient sample to investigate HTLV-1 infection in Campo Grande, Central Brazil. The 0.7% (95% CI: 0.4–0.9) prevalence rate of HTLV-1 infection found in this study was similar to that observed among Brazilian blood donors (0.48%), as well as in an isolated Afro-Brazilian slave-descendant community in Central Brazil (0.5%) and in MSM from Campinas, Southeast Brazil (1.5%; 95% CI: 0.5–3.0).4, 6, 13 Nevertheless, the prevalence rate found in pregnant women in Mato Grosso do Sul State (0.11%) was lower than that reported in this study.14
According to several risk behaviors assessed in this study, mainly through sexual exposures, there is a high vulnerability for STIs acquisition in these individuals. Significant differences in relation to commercial sex worker were found between HTLV-1-infected and non-infected MSM. Greater vulnerability and social marginalization was observed in this group of population.8, 10 Furthermore, MSM who exchanged money for sex present a greater risk for acquiring STIs than female sex workers and other non-sex workers MSM due to combination of high risk behaviors, such as having multiple sexual partners, unprotected sexual intercourse and receptive anal intercourse.8, 15
Regarding MSM population, greater number of lifetime sex partners is a high-risk behavior factor for HTLV-1 infection according to some studies.7, 16 A cross-sectional surveillance study for HIV among MSM from ten different Brazilian cities reported multiple sexual partners and inconsistent use of condom with casual and commercial partners.9 Additionally, others studies conducted in different countries have pointed rectal mucosal injury during intercourse as a risk factor for HTLV-1 and other STIs in this population.7, 16, 17
In the past, blood transfusion was an important route of HTLV-1 transmission, with a seroconversion rate ranging from 40 to 60%.1 In 1993, serological screening for HTLV-1/2 became mandatory in Brazilian blood banks, interrupting this transmission route. History of blood transfusion before 1993 was found in one infected MSM (ID-13), as shown in Table 1. Although results are suggestive of HTLV transmission via transfusion, the three infected MSM may have acquired the infection through sexual intercourse, given they were sex workers, a group known to be at risk for acquiring STIs.4, 8, 15
The positive samples were classified as belonging to the Transcontinental (A) subgroup of the Cosmopolitan (a) subtype. This subgroup of HTLV-1 is considered the most prevalent in Brazil.3, 18 Phylogenetic analysis showed that HTLV-1 strains isolated from the present study were found within the Brazilian and South American clusters. In addition, two HTLV-1 sequences out of Latin American countries, one sequence from an Indian commercial sex worker and other from a Portuguese injecting drug user (IDU) clustered with the study strains.
One of the positive samples was amplified only in the tax region of HTLV-1 (HSH28), probably due to the low HTLV-1 proviral load, since that tax region is more readily PCR amplified than other HTLV-1 regions. According to previous studies, the high sensitivity and wide dynamic range of HTLV-1 tax sequence detection allow for determination of a broad range of HTLV-1 proviral loads.19, 20
Some limitations found in this study should be considered. First, related to the veracity of the study subjects’ responses about risk behaviors. Second, MSM from this study were recruited from gay-related public and private places, who may not be representative of the whole MSM community. Furthermore, a convenience sample was selected because the method respondent-driven sampling (RDS) could not be implemented for recruitment of this study population. Third, the small number of HTLV-1 cases may have caused a limited power to evaluate some statistical associations of risk. In addition, vertical transmission of HTLV-1 infection among the studied MSM could not be analyzed for lack of information about this route. Despite these limitations, the study provides important information about HTLV-1 infection among MSM group in Central Brazil.
In summary, the prevalence of HTLV-1 infection in MSM was similar to the prevalence among Brazilian blood donors. However, the high sexual risk behavior found in this study population makes them more susceptible to acquiring HTLV-1 infection than Brazilian blood donors in general. The HTLV-1 subtype identified in the studied individuals is consistent with those previously found in Brazil. Therefore, educational measures for controlling the spread of HTLV-1 and other STIs in this population are needed and could be achieved through the implementation of specific preventive activities among MSM in Central Brazil.
Ethics approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grant/Award Number: 134191/2012-9; and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT/MS), Grant/Award Number: 23/200.283/2009.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors acknowledge all the MSM who participated in this study and the important contribution of entities’ members that collaborated with this project: Reference Center for Human Rights in the Prevention and Combat of Homophobia (CentrHo) and Mato Grosso do Sul State Association of Travestites and Transsexuals (ATMS-MS).
References
- 1.Proietti F.A., Carneiro-Proietti A.B., Catalan-Soares B.C., Murphy E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 2.Nunes D., Boa-Sorte N., Grassi M.F.R., et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS One. 2017;12:e0171303. doi: 10.1371/journal.pone.0171303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gessain A., Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalan-Soares B., Carneiro-Proietti A.B., Proietti F.A. Interdisciplinary HTLV Research Group Heterogeneous geographic distribution of human T-cell lymphotropic viruses I and II (HTLV-I/II): serological screening prevalence rates in blood donors from large urban áreas in Brazil. Cad Saude Publica. 2005;21:926–931. doi: 10.1590/s0102-311x2005000300027. [DOI] [PubMed] [Google Scholar]
- 5.Bandeira L.M., Uehara S.N., Asato M.A., et al. High prevalence of HTLV-1 infection among Japanese immigrants in non-endemic area of Brazil. PLoS Negl Trop Dis. 2015;9:e0003691. doi: 10.1371/journal.pntd.0003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soares C.C., Georg I., Lampe E., et al. HIV-1, HBV, HCV, HTLV, HPV-16/18, and Treponema pallidum infections in a sample of Brazilian men who have sex with men. PLoS One. 2014;9:e102676. doi: 10.1371/journal.pone.0102676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Rosa A.M., Zunt J.R., Peinado J., et al. Retroviral infection in Peruvian men who have sex with men. Clin Infect Dis. 2009;49:112–117. doi: 10.1086/599609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuylsteke B., Semde G., Sika L., et al. High prevalence of HIV and sexually transmitted infections among male sex workers in Abidjan Cote d’Ivoire: need for services tailored to their needs. Sex Transm Infect. 2012;88:288–293. doi: 10.1136/sextrans-2011-050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr L.R., Mota R.S., Kendall C., et al. HIV among MSM in a large middle-income country. AIDs. 2013;27:427–435. doi: 10.1097/QAD.0b013e32835ad504. [DOI] [PubMed] [Google Scholar]
- 10.Rocha G.M., Kerr L.R.F.S., Kendall C., Guimarães M.D.C. Risk behavior score: a practical approach for assessing risk among men who have sex with men in Brazil. Braz J Infect Dis. 2018;22:113–122. doi: 10.1016/j.bjid.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins R.M., do Nascimento L.B., Carneiro M.A., et al. HTLV-1 intrafamilial transmission through three generations in an isolated Afro-Brazilian community. J Clin Virol. 2010;48:155–157. doi: 10.1016/j.jcv.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa Y., Yamashita M., Usuku K., Izumo S., Nakagawa M., Osame M. Phylogenetic subgroups of human T cell lymphotropic virus (HTLV) type I in the tax gene and their association with different risks for HTLV-I-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2000;182:1343–1349. doi: 10.1086/315897. [DOI] [PubMed] [Google Scholar]
- 13.Nascimento L.B., Carneiro M.A., Teles S.A., et al. Prevalence of infection due to HTLV-1 in remnant quilombos in Central Brazil. Rev Soc Bras Med Trop. 2009;42:657–660. doi: 10.1590/s0037-86822009000600009. [DOI] [PubMed] [Google Scholar]
- 14.Dal Fabbro M.M., Cunha R.V., Bóia M.N., et al. HTLV 1/2 infection: prenatal performance as a disease control strategy in State of Mato Grosso do Sul. Rev Soc Bras Med Trop. 2008;41:148–151. doi: 10.1590/s0037-86822008000200003. [DOI] [PubMed] [Google Scholar]
- 15.Belza M.J. Risk of HIV infection among male sex workers in Spain. Sex Transm Infect. 2005;81:85–88. doi: 10.1136/sti.2003.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zunt J.R., La Rosa A.M., Peinado J., et al. Risk factors for HTLV-II infection in Peruvian men who have sex with men. Am J Trop Med Hyg. 2006;74:922–925. [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt A.J., Rockstroh J.K., Vogel M., et al. Trouble with bleeding: risk factors for acute hepatitis C among HIV-positive gay men from Germany – a case-control study. PLoS One. 2011;6:e17781. doi: 10.1371/journal.pone.0017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magri M.C., Brigido L.F., Rodrigues R., Morimoto H.K., Ferreira J.L., Caterino-de-Araujo A. Phylogenetic and similarity analysis of HTLV-1 isolates from HIV-coinfected patients from the south and southeast regions of Brazil. AIDs Res Hum Retroviruses. 2012;28:110–114. doi: 10.1089/aid.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrams A., Akahata Y., Jacobson S. The prevalence and significance of HTLV-I/II seroindeterminate western blot patterns. Viruses. 2011;3:1320–1331. doi: 10.3390/v3081320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soldan S.S., Graf M.D., Waziri A., et al. HTLV-I/II seroindeterminate western blot reactivity in a cohort of patients with neurological disease. J Infect Dis. 1999;180:685–694. doi: 10.1086/314923. [DOI] [PubMed] [Google Scholar]