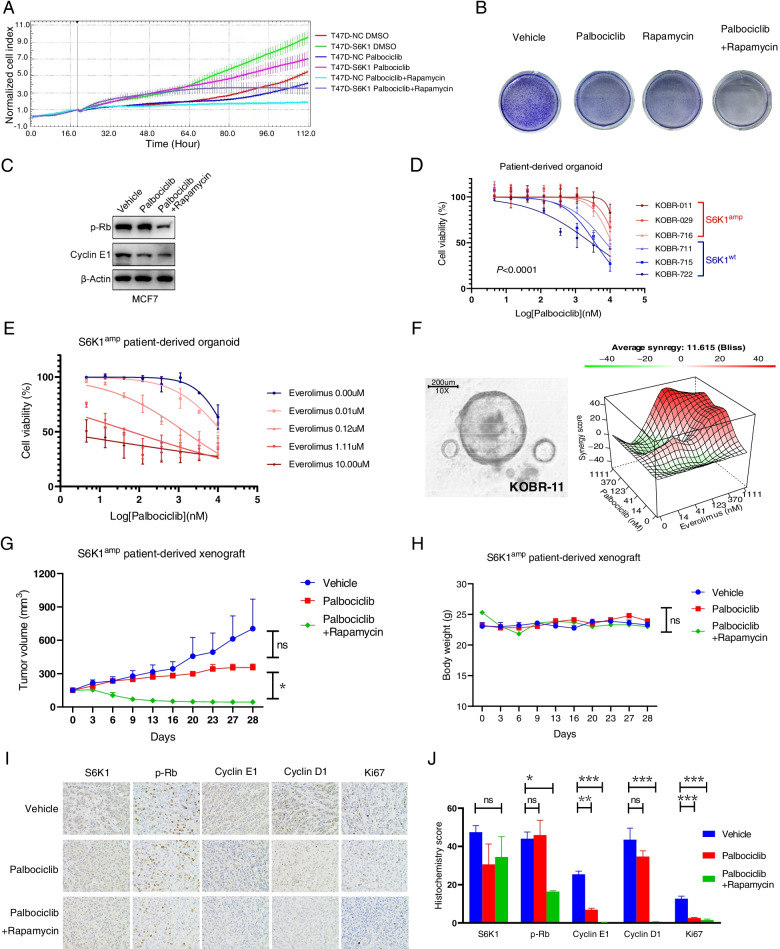
Fig. 6.
mTOR inhibitors can reverse resistance to palbociclib in vitro and in vivo. A xCELLigence system analysis of the proliferation of exogenous S6K1-expressed T47D cells or control cells treated with either vehicle, palbociclib (100 nM) or palbociclib (100 nM) plus mTOR inhibitor rapamycin (500 nM). B Clonogenic assay of MCF-7 cells treated with either vehicle, palbociclib (5 μM), rapamycin (1 μM) or combination. C The expression of cyclin E1 and p-Rb in MCF-7 cells treated as described in B was detected by western blotting. β-Actin was used as the loading control. D Three breast cancer patient-derived organoids (PDOs) with S6K1 amplification (KOBR-011, − 029, − 716) were identified and confirmed to be resistant to palbociclib. KOBR-711, − 715, and − 722 with wild type S6K1 were used as negative controls. E Addition of mTOR inhibitor everolimus to palbociclib showed greater inhibition of cell viability in the S6K1-amplificated PDO. F The landscape of the combination responses for palbociclib plus everolimus in the S6K1-amplificated PDO (KOBR-011) via the Bliss model. G In vivo tumour-growth measurement of S6K1-amplificated patient derived xenografts treated with either vehicle, palbociclib or palbociclib plus rapamycin for 28 days. Data are presented as the mean ± SEM; P value was calculated by Mixed-effects model. ns, not significant. *, P < 0.05. H Dynamic changes in body weight of mice as described in G. Data are presented as the mean ± SEM; P value was calculated by Mixed-effects model. ns, not significant. I, J Immunohistochemistry analysis of mouse tumour slices with the indicated antibodies. I Representative images. J Quantitative analysis. Data are presented as the mean ± SEM. P value was calculated by one-way ANOVA. ns, not significant. *, P < 0.05, **, P < 0.01, ***, P < 0.001