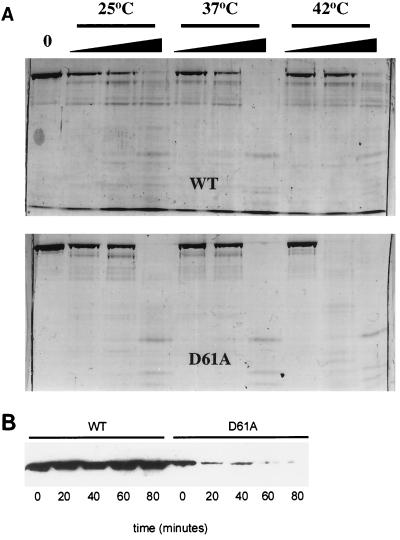
FIG. 2.
Stability of ς70D61A in vitro and in vivo. (A) Limited trypsinolysis. ς70D61A (2 μg) was subjected to trypsin digestion at three temperatures, as indicated, to assess possible structural defects. Wedges indicate increasing trypsin concentration (0.0125, 0.0625, and 0.025 μg). Digestion of wild-type (WT) ς70 is shown for reference in the top panel. Fragments were resolved on a sodium dodecyl sulfate–8% polyacrylamide gel and visualized with Coomassie brilliant blue staining. (B) Immunoblot analysis of culture lysates of strain 19284 (wild-type [WT] ς70 and ς70D61A). Exponentially growing cells at 37°C were upshifted to 44°C. Lysates were prepared from cells harvested at the indicated time points following temperature upshift, and proteins were resolved on a sodium dodecyl sulfate–8% polyacrylamide gel. Following Western transfer, histidine-tagged ς70 proteins were detected by using a six-His-tagged monoclonal antibody (Clontech).