FIG 2.
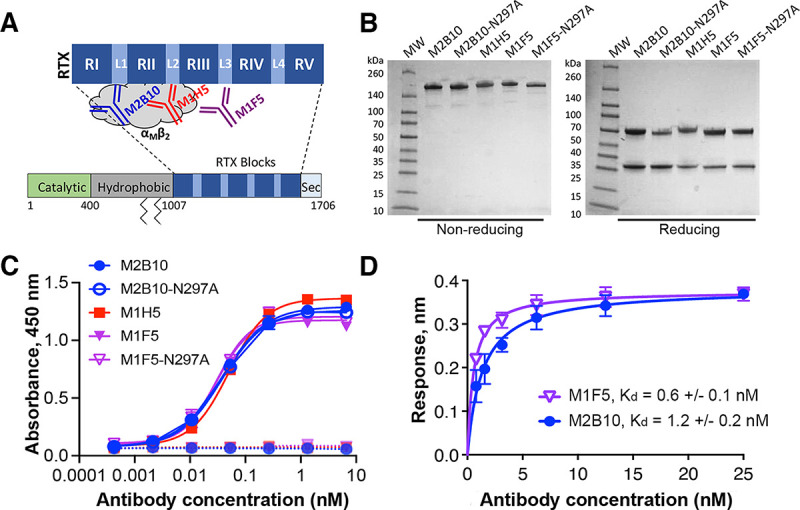
Antibodies binding different RTX epitopes have similar biophysical characteristics. (A) Schematic of the ACT domain structure showing the antibody binding sites. Mature ACT consists of a catalytically active N-terminal domain (green), a central hydrophobic domain with acylation sites at residues 860 and 983 (gray), an RTX domain comprised of five repeat blocks (RI to RV) joined by linkers (L1 to L4), and a C-terminal secretion signal (Sec). (B) SDS-PAGE gel of purified antibodies under nonreducing and reducing conditions, with molecular weight (MW) markers shown. (C) Antibody binding to wells coated with RTX751 (1 μg/mL) (solid lines) or uncoated wells (dashed lines) was assessed by an ELISA with anti-mouse Fc–HRP detection. The binding data were fit to a four-parameter logistic curve in GraphPad Prism. Shown are the averages from duplicates, with error bars representing the data range; the experiment was repeated twice. (D) Binding of M2B10 and M1F5 to RTX751 via biolayer interferometry. Antibodies were immobilized on anti-mouse Fc sensors and dipped into RTX751 (six concentrations from 25 to 0.8 nM) for a 30-min equilibration. Binding affinity was determined by data fitting to a Langmuir isotherm using Octet Red96 instrument software (FortéBio); the Kd values are shown as the means and ranges from two replicate experiments.
