FIG 3.
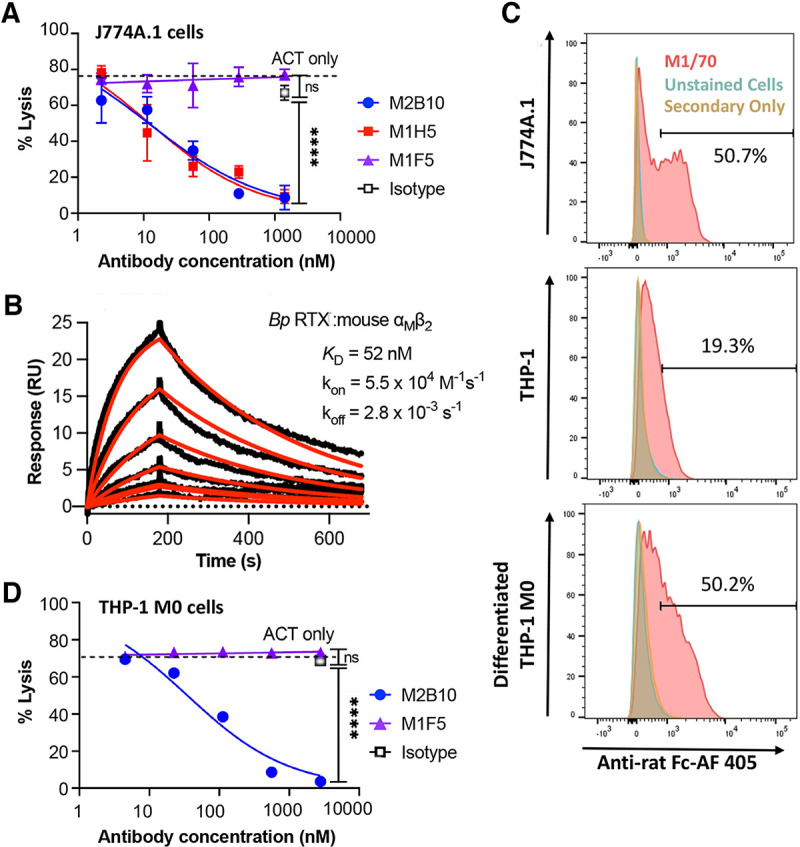
Neutralizing anti-ACT antibodies protect mouse and human macrophages against ACT cytotoxicity in vitro. (A) Antibody neutralization of ACT cytotoxicity was assessed with mouse macrophage J774A.1 cells. ACT (5 μg/mL) was preequilibrated with antibody (serially diluted from a 50-fold molar excess over ACT) and added to J774A.1 cells (5 × 105 cells/mL) for 2 h at 37°C. Cell lysis was measured via lactate dehydrogenase release, normalized to untreated and surfactant-treated control cells to report the percentage of cell lysis. The experiment was repeated twice, with technical triplicates; error bars indicate the standard errors from duplicate assays. Reported IC50 values for each experiment were determined by four-parameter logistic fits in GraphPad. (B) Binding kinetics and equilibrium dissociation constants for immobilized RTX751 and soluble, purified mouse αMβ2 integrin (2-fold dilutions from 200 to 6.1 nM) measured on a Biacore X100 instrument and analyzed using BIAevaluation software. Data are shown in black; model fits are shown in red. (C) Surface αMβ2 levels on J774A.1 cells and undifferentiated and differentiated THP-1 cells were determined by flow cytometry with rat anti-αMβ2 antibody M1/70 followed by anti-rat Fc–Alexa Fluor 405 (AF 405) secondary antibody. To differentiate THP-1 cells, the cells (3 × 105 cells/mL) were treated with 10 ng/mL of PMA for 24 h and then allowed to rest for 72 h in PMA-free medium. The percentages of cells with the same levels of fluorescence are indicated. (D) Antibody neutralization of ACT in M0 differentiated human THP-1 cells, performed as described above for panel A but with 10 μg/mL ACT to achieve ~75% cell lysis. ns, not significant. For panels A and C, the averages and standard errors for triplicate technical replicates are shown; the experiment was repeated twice.
