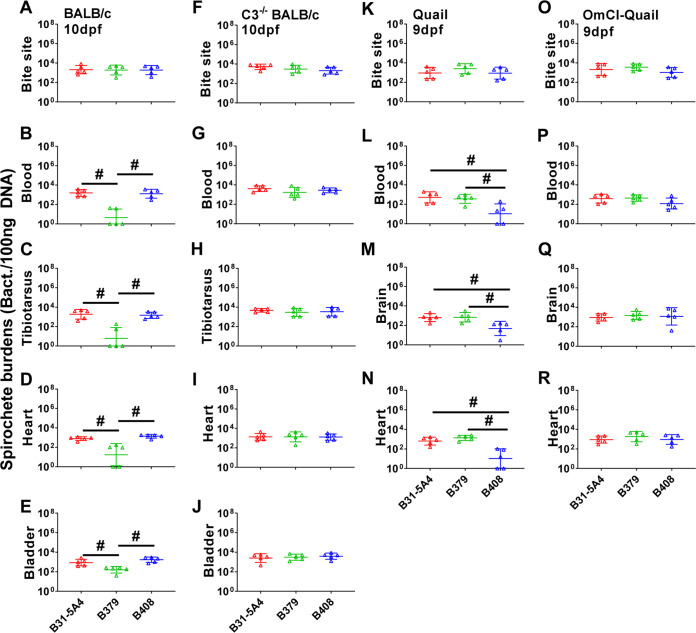
FIG 4.
B. burgdorferi exhibits host- and bacterial genotype-specific early dissemination in a complement-dependent fashion. The I. scapularis nymphs carrying B. burgdorferi strains B31-5A4, B379, or B408 were allowed to feed until they were replete on five wild-type (A to E) or C3-deficient BALB/c (C3−/− BALB/c) (F to J) mice or wild-type (K to N) or O. moubata complement inhibitor [OmCI]-treated (O to R) quail. The mice and quail were euthanized at 10 and 9 days after nymph feeding (dpf), respectively. The bacterial loads at the site where nymphs fed (bite site) (A, F), blood (B, G), tibiotarsus joints (C, H), heart (D, I), and bladder of mice (E, J) and the site of nymphs bite (bite site) (K, O), blood (L, P), brain (M, Q), and heart (N, R) of quail collected immediately after euthanasia were determined by quantitative PCR [qPCR]. The bacterial loads in tissues or blood were normalized to 100 ng total DNA. Shown are the geometric means of bacterial loads ± geometric standard deviation of five mice or quail per group. There were significant differences (P < 0.05, the Kruskal-Wallis test followed by the two-stage step-up method of Benjamini, Krieger, and Yekutieli) in the spirochete burdens between two strains relative to each other (#).