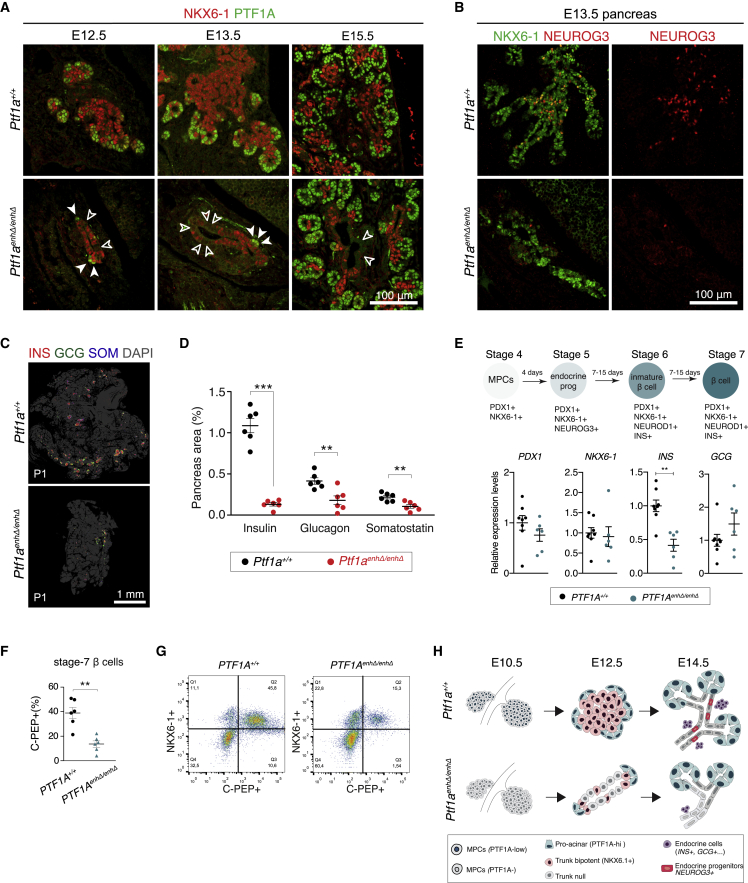
Figure 5.
PTF1A in MPCs primes endocrine differentiation of mouse bipotent trunk progenitors
(A) E12.5-15.5 pancreas showing NKX6-1 (red) in “trunk” bipotent duct-endocrine progenitors, and PTF1A (green) in peripheral pro-acinar cells. White empty arrows point to NKX6-1 negative poorly differentiated trunk cells in Ptf1aenhΔ/enhΔ pancreas. White solid arrowheads depict PTF1A-positive tip cells in Ptf1aenhΔ/enhΔ pancreas.
(B) NEUROG3+ endocrine progenitors (red) are severely reduced in E13.5 Ptf1aenhΔ/enhΔ pancreas (see also Figure S5J).
(C and D) Insulin (INS), glucagon (GCG), and somatostatin (SOM) immunofluorescence of neonatal (P1) and E18.5 pancreas showed reduced endocrine cells. A representative section from P1 is shown in (C), whereas (D) shows quantifications of the relative pancreas area occupied by each endocrine cell type in E18.5 (n = 6/genotype; ∗∗p ≤ 0.01, ∗∗∗Welch’s t-test p ≤ 0.0001).
(E) qRT-PCR of endocrine markers in human hPSC-derived beta-like cells (n = 6–8 independent differentiations/genotype, using 6 PTF1AenhΔ/enhΔ and 4 PTF1A+/+ control lines). Error bars represent mean ± SEM. Mann-Whitney test, ∗∗p < 0.01.
(F and G) (F) Flow cytometry for C-peptide expressing beta-like cells in differentiated control and mutant S7 stem cell islets (Mann-Whitney test, ∗∗p < 0.01), and (G) representative FACS plots (n = 6 independent differentiations/genotype).
(H) Schematic summarizing the differentiation phenotype in Ptf1aenhΔ/enhΔ pancreas. See also Figure S5.