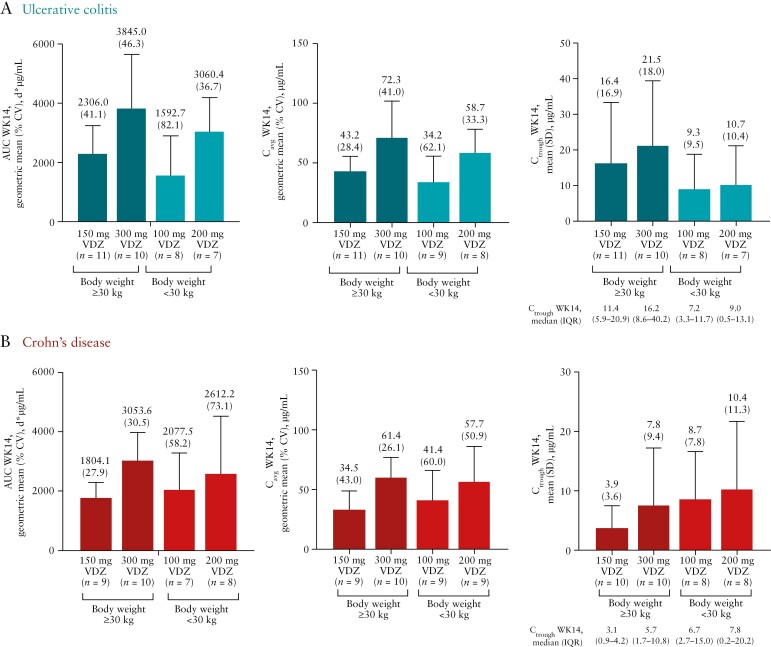
Figure 2.
Pharmacokinetic parameters of VDZ AUC, Cavg and Ctrough at Week 14 in patients with [A] ulcerative colitis and [B] Crohn’s disease [pharmacokinetic analysis set]. N = 88; one patient was randomized but not treated. The pharmacokinetic analysis set was defined as all patients who received at least one dose of VDZ and had at least one measurable concentration of VDZ. AUC, area under the serum concentration curve; Cavg, average concentration; Ctrough, observed serum concentration at the end of the dosing interval; CV, coefficient of variation; IQR, interquartile range; SD, standard deviation; VDZ, vedolizumab; WK, Week.