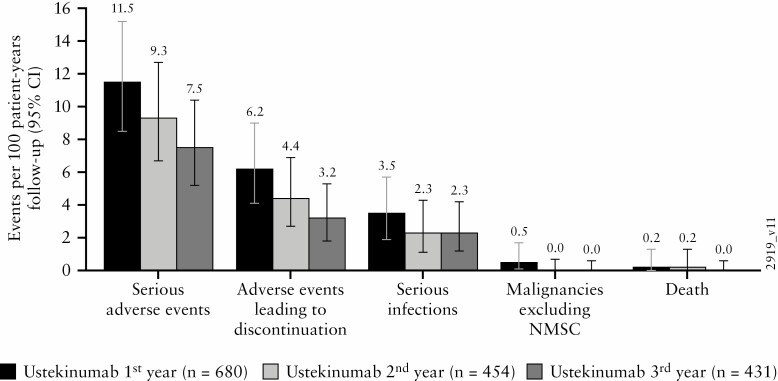
Figure 7.
Key safety events per 100 patient-years of exposure during the first, second, and third year of ustekinumab maintenance therapy. AE, adverse events; CI, confidence interval; IV, intravenous; LTE, long-term extension; NMSC, nonmelanoma skin cancer; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous.a. Number of AEs per 100 patient-years of follow-up and 95% confidence interval [rates by each year of follow-up] in the pooled ustekinumab ulcerative colitis safety cohort. Confidence intervals based on an exact method assuming that the observed number of events follows a Poisson distribution.
b. Infection as assessed by the investigator.
c. All ustekinumab [first year] includes: 1] patients who received ustekinumab SC [q8w or q12w] in this maintenance study; 2] patients who were in clinical response to ustekinumab IV induction dosing and received placebo SC on entry into this maintenance study; and 3] data from Week 0 to Week 8 for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to placebo SC on entry into this maintenance study.
d. All ustekinumab-treated in the LTE [second year] includes: 1] data from Week 44 through Week 96 for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to ustekinumab 90 mg SC q12w on entry into the maintenance study; 2] patients who were in clinical response to ustekinumab IV induction dosing and were randomised to receive ustekinumab 90 mg SC q8w on entry into the maintenance study, with data from Week 44 through Week 96; 3] patients who were in clinical response to ustekinumab IV induction dosing, randomised to receive placebo SC on entry into the maintenance study, and had a dose adjustment prior to Week 96 to ustekinumab 90 mg SC q8w, with data from the time of dose adjustment onward; and 4] patients who were not in clinical response to ustekinumab at induction Week 8 but were in clinical response at induction Week 16 after an SC administration of ustekinumab at induction Week 8 and received ustekinumab 90 mg SC q8w on entry into the maintenance study with data from Week 44 through Week 96.
e. All ustekinumab in the LTE [third year] includes: 1] data from Week 96 through Week 156 for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to ustekinumab 90 mg SC q12w on entry into the maintenance study; 2] patients who were in clinical response to ustekinumab IV induction dosing and were randomised to receive ustekinumab 90 mg SC q8w on entry into the maintenance study, with data from Week 96 through Week 156; 3] patients who were in clinical response to ustekinumab IV induction dosing, randomised to receive placebo SC on entry into the maintenance study, and had a dose adjustment at or after Week 96 to ustekinumab 90 mg SC q8w, with data from the time of dose adjustment onward; and 4] patients who were not in clinical response to ustekinumab at induction Week 8 but were in clinical response at induction Week 16 after an SC administration of ustekinumab at induction Week 8 and received ustekinumab 90 mg SC q8w on entry into the maintenance study with data from Week 96 through Week 156.