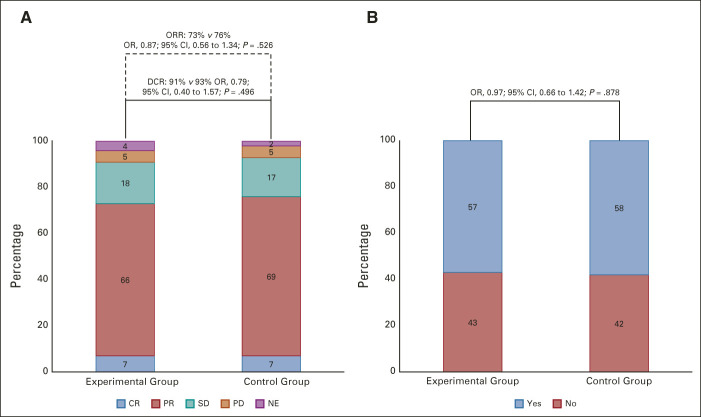
FIG 2.
Response parameters according to the treatment arm: (A) ORR and disease control rate and (B) early tumor shrinkage. The control group indicates FOLFOX plus panitumumab. The experimental group indicates mFOLFOXIRI plus panitumumab. CR, complete response; DCR, disease control rate; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; mFOLFOXIRI, modified fluorouracil, leucovorin, oxaliplatin, and irinotecan; NE, not evaluable; OR, odds ratio; ORR, objective response rate; PD, progression disease; PR, partial response; SD, stable disease.