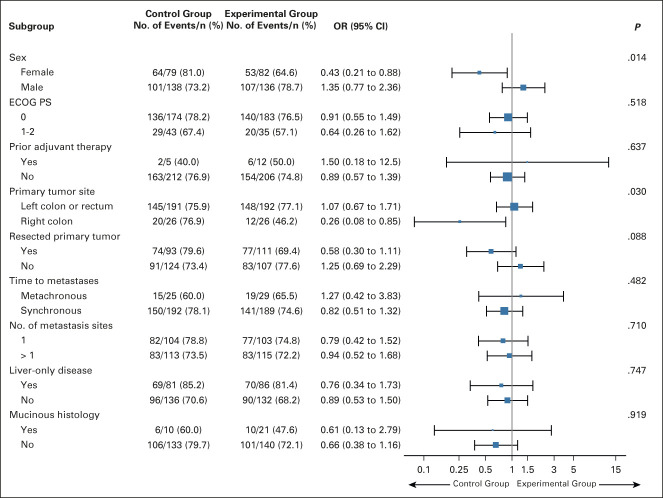
FIG 3.
Subgroup analyses of the objective response rate according to clinical characteristics of the intention-to-treat population. The control group indicates FOLFOX plus panitumumab; the experimental group indicates mFOLFOXIRI plus panitumumab. ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; mFOLFOXIRI, modified fluorouracil, leucovorin, oxaliplatin, and irinotecan; OR, odds ratio.