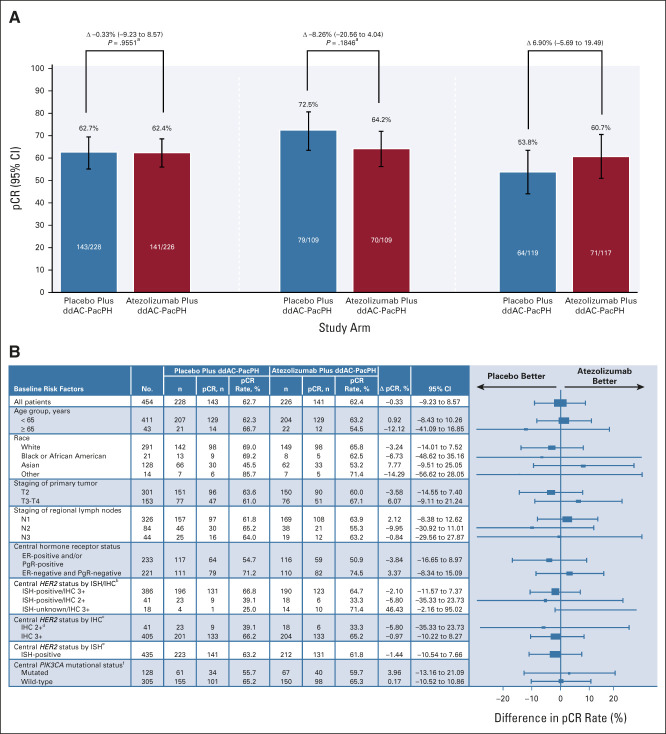
FIG 2.
(A) pCR in the ITT (primary end point), PD-L1–positive (primary end point), and PD-L1–negative (secondary end point) populations, and (B) pCR in subgroups of the ITT population. aStratified (Cochran-Mantel-Haenszel test). bISH-positive/IHC 0/1+: number of pCR events in placebo/atezolizumab: n = 0/2 versus n = 0/2. ISH-positive/IHC unknown: n = 1/2 versus n = 2/2. ISH-negative/IHC 3+: n = 1/1 versus n = 0/0. cIHC 0/1+: number of pCR events in placebo/atezolizumab: n = 0/2 versus n = 0/2. IHC unknown: n = 1/2 versus n = 2/2. dPatients whose tumor test results were IHC 2+ had ISH-positive status. eISH-negative: number of pCR events in placebo/atezolizumab: n = 1/1 versus n = 0/0. ISH-unknown: n = 1/4 versus n = 10/14. fPIK3CA missing: number of pCR events in placebo/atezolizumab: n = 14/21 versus n = 11/20. ddAC, dose-dense doxorubicin and cyclophosphamide; ER, estrogen receptor; H, trastuzumab; IHC, immunohistochemistry; ISH, in situ hybridization; ITT, intention-to-treat; N, nodal stage; NE, not estimable; P, pertuzumab; Pac, paclitaxel; pCR, pathologic complete response (ypT0/is ypN0); PD-L1, programmed cell death-ligand 1; PgR, progesterone receptor; T, tumor stage.