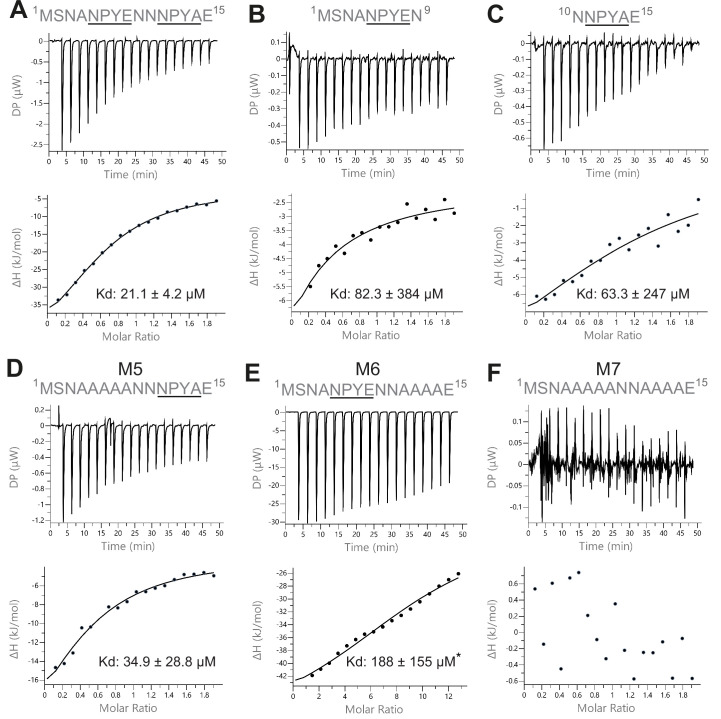
Figure 3. Isothermal titration calorimetry (ITC) measurements of the interaction between Sec3 and variant versions of the NPY motifs of Sso2.
(A) Wild type double NPY motifs of Sso2 bound Sec3 with a dissociation constant (Kd) of approximately 21 µM. (B and C) Either of the two NPY motifs alone bound Sec3 much more weakly than the two together, with Kd values increased by three- to fourfold. (D and E) Mutation of either NPY motif (i.e. M5 and M6) also substantially reduced the binding affinity of Sso2 to Sec3. (F) Double mutation (M7) of both NPY motifs completely abolished the interaction between Sso2 and Sec3. For M6 in (E), the peptide concentration had to be increased by sixfold to detect its very weak interaction with Sec3.