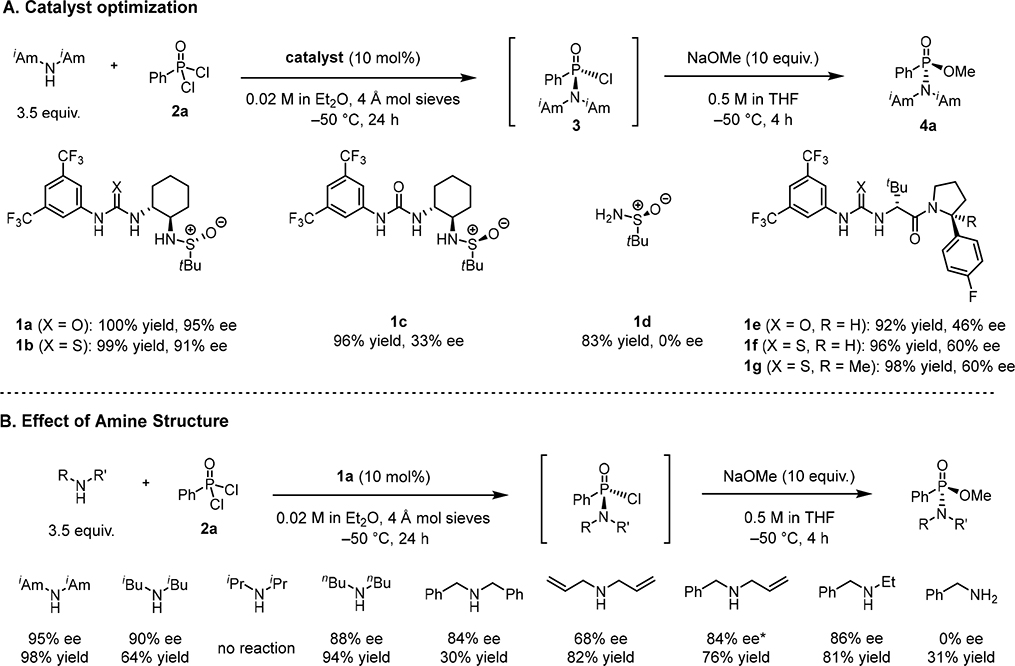
Figure 2. Optimization studies.
Yield values reflect product quantification by 31P NMR relative to an internal standard. Reactions were carried out using a one-pot procedure without purification of 3. Concentration values correspond to the initial concentration of the limiting stoichiometric reagent. (A) Catalyst optimization for enantioselective reaction of diisoamylamine with phenyl phosphonic dichloride. Reactions were carried out on a 0.06 mmol scale. (B) Optimization of amine structure for enantioselective substitution reaction with phenyl phosphonic dichloride. Reactions were carried out on a 0.06 mmol scale. *Reaction performed at −40 °C for 48 h.