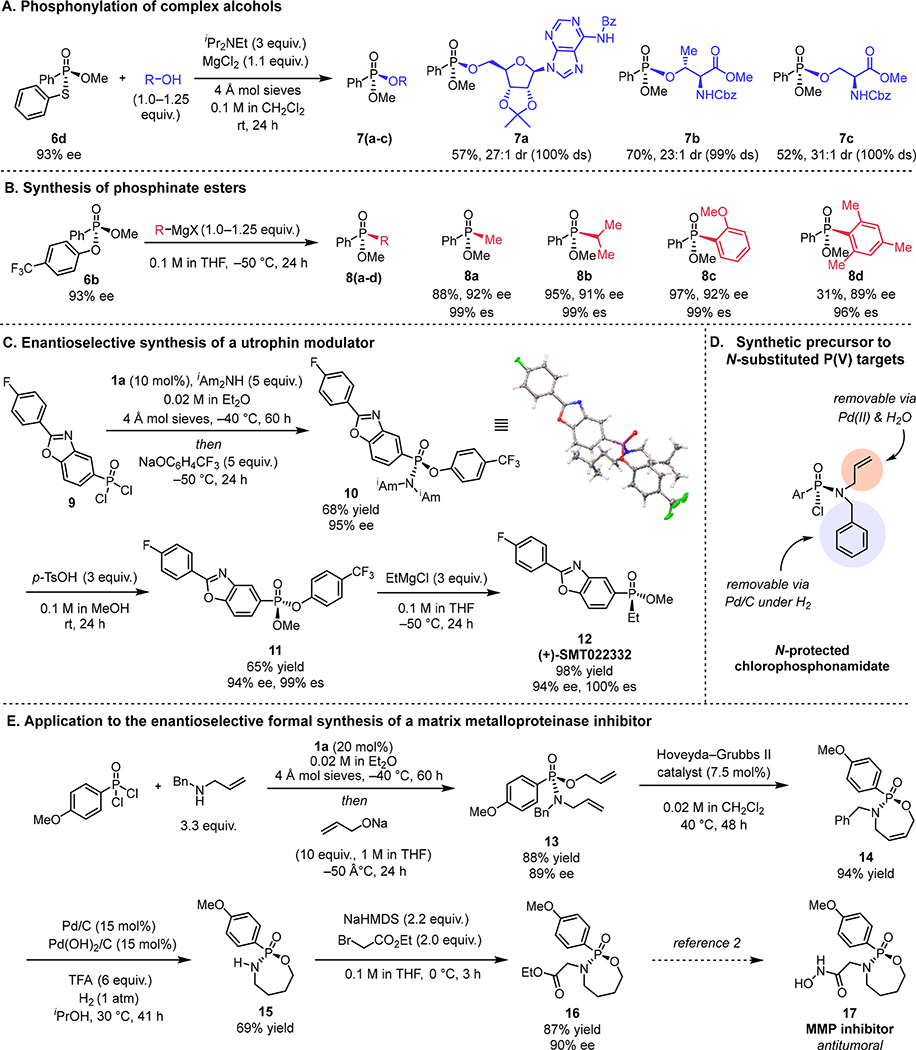
Fig. 4. Application to the synthesis of chiral P(V) targets.
All yield values refer to chromatographically purified, isolated products. Concentration values correspond to the initial concentration of the limiting stoichiometric reagent. (A) Stereospecific phosphonylation of precious alcohols with 6d. Reactions were carried out on 0.1 mmol scale. (B) Stereospecific addition of Grignard reagents to 6b for the synthesis of enantioenriched phosphinate esters. Absolute stereochemistry of 8a was determined by comparison of optical rotation to literature value; others assigned by analogy. Reactions run on 0.05–0.1 mmol scale. *Prepared from 6b that was in 92% ee. (C) Application of method to the enantioselective synthesis of (+)-SMT022332. Yield values refer to isolated yields. Absolute stereochemistry of 10 assigned by the depicted X-ray crystal structure, and of 12 by comparison of the optical rotation to the literature value. (D) Orthogonally N-protected chlorophosphonamidate. (E) Formal synthesis of a matrix metalloproteinase inhibitor. TFA = trifluoroacetic acid.