Figure 1. Fitness advantage of cancer-driving mutations enables the creation of a progressive series of genome-edited, human cancer models.
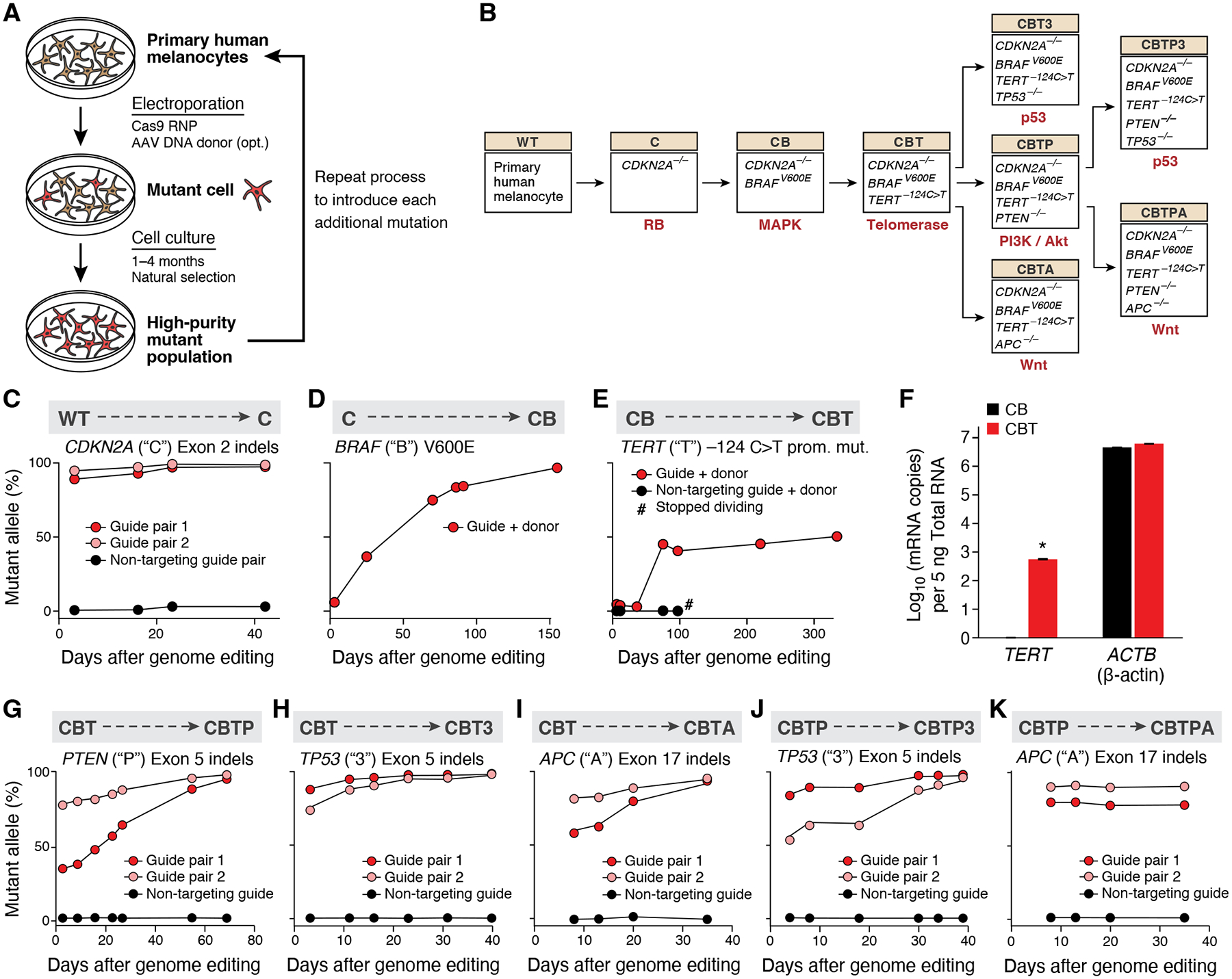
(A) Experimental approach for introducing sequential melanoma mutations into the genomes of primary human melanocytes using CRISPR/Cas9. RNP: ribonucleoprotein. AAV: adeno-associated virus. (B) Editing tree. The nine isogenic models of melanoma generated (boxes), the perturbed genes in each model (inside box), the genotype abbreviation (beige boxes), and the molecular pathway dysregulated by the most recent genome edit (red text). (C-E) Sequential introduction of first three mutations by CRISPR/Cas9 genome editing of wild-type (‘WT’) melanocytes. (C) First mutation: CDKN2A (‘C’). (D) Second mutation: BRAF (‘B’). (E) Third mutation: TERT (‘T’). TERT editing confers replicative immortality to CB melanocytes. Allele frequencies of each engineered mutation (y axis) shown over time (x axis). #: measurement of allele frequency discontinued due to cell senescence. (F) Addition of the −124C>T TERT promoter mutation activates TERT expression. Mean of log 10 number of TERT and β-actin (ACTB) transcripts (y axis) measured by qPCR in CB (black) and CBT (red) cells. Error bars: SD. n=3. *: p < 0.01, one-tailed, one-sample Student’s t-test. (G-I) Introduction of fourth mutation into CBT melanocytes. (G) Allele frequencies of knockout of PTEN (‘P’), (H) knockout of TP53 (‘3’), and (I) knockout of APC (‘A’). (J-K) Introduction of fifth mutations into CBTP melanocytes (J) Allele frequency of knockout of PTEN and (K) knockout of TP53. Allele frequencies (y axis) shown over time (x axis), as assessed by indels in the respective loci in genomic DNA.
