Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to inhibit pyroptosis and apoptosis, which play important roles in the development and progression of contrast-induced acute kidney injury (CI-AKI). However, to the best of our knowledge, no studies have investigated the potential effect of PCSK9 inhibitors on the prevalence of CI-AKI after percutaneous coronary intervention (PCI). This study aimed to determine whether PCSK9 inhibitors are associated with the prevalence of CI-AKI. The medical records of 309 (mean age, 63.35 years; 71.84% male) patients with acute myocardial infarction who underwent PCI at our institution were retrospectively analyzed. Overall, 149 and 160 patients were assigned to the evolocumab and control groups, respectively. Serum creatinine levels were examined preoperatively and 24–72 h postoperatively and compared between groups. Data were grouped according to the occurrence of CI-AKI, and a univariate analysis was conducted to exclude suspected influencing factors that led to CI-AKI occurrence. After adjusting for confounding factors, a logistic regression analysis was performed to assess the association between evolocumab administration (independent variable) and CI-AKI occurrence (dependent variable). The prevalence of CI-AKI was significantly lower in the evolocumab group (6.7%) than in the control group (20.0%; p < 0.01).We further evaluated the correlation between exposure factor and outcome. The relative risk(RR) between the use of evolocumab and the occurrence of CI-AKI was 0.34(95% CI 0.17-0.66,p<0.01).This result indicate a significant association between the use of evolocumab and a reduction in the incidence of CI-AKI.The logistic regression analysis results revealed that evolocumab was significantly associated with CI-AKI. The use of PCSK9 inhibitors, hydration therapy, and statin administration appears promising for preventing CI-AKI in patients with acute myocardial infarction undergoing PCI.
1. Introduction
Coronary interventional procedures using intravascular contrast media are extensively performed worldwide. However, contrast-induced acute kidney injury (CI-AKI), a serious complication resulting from intravascular contrast media, occurs with an incidence of 10–15% during cardiac interventions and can reach 50% in high-risk populations [1]. Hydration therapy cannot prevent CI-AKI in high-risk patients [2]; therefore, identifying safe and effective methods to prevent CI-AKI is particularly important.
The Further Cardiovascular Outcomes Research with Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibition in Patients with Elevated Risk (FOURIER) trial, Open-Label Study of Long-Term Evaluation against LDL-C (OSLER-1) trial, and Evolocumab for Early Reduction of LDL-Cholesterol Levels in Patients with Acute Coronary Syndromes (EVOPACS) study recommended PCSK9 inhibitors for the treatment of atherosclerotic cardiovascular disease [3–5]. As a type of PCSK9 inhibitor, evolocumab was administered on a large scale to treat acute coronary syndrome (ACS) [5]. PCSK9 inhibitors have been shown to inhibit pyroptosis and apoptosis [6–8], which play important roles in the development and progression of CI-AKI [9–11]. However, to the best of our knowledge, no studies have investigated the potential effect of PCSK9 inhibitors on the prevalence of CI-AKI after percutaneous coronary intervention (PCI).
This study aimed to investigate whether the administration of the PCSK9 inhibitor evolocumab combined with statin therapy in patients with acute myocardial infarction can reduce the prevalence of CI-AKI better than statin therapy alone.
2. Materials and Methods
2.1. Study Population
This study was a retrospective analysis of the medical files of 309 patients with acute myocardial infarction who underwent PCI at Tianjin Chest Hospital between January 2019 and December 2019. This study enrolled 222 male and 87 female patients with an average age of 63.35 (standard deviation (SD): 10.62) years.
The inclusion criteria for this study were as follows: male or female patients aged 18–90 years, ultra-high-risk atherosclerotic cardiovascular disease patients who met the diagnostic criteria for acute myocardial infarction, and patients receiving coronary intervention, aspirin combined with ticagrelor or clopidogrel, or treatment with atorvastatin or rosuvastatin, consistent with the indication for evolocumab.
We excluded 35 patients who met the following criteria: renal dysfunction, active renal disease or a need for renal replacement before the intervention, contraindications to the administration of PCSK9 inhibitors, active infection, malignancy, severe liver dysfunction, acute stroke, abnormal thyroid function, and Killip class >II cardiogenic shock.
The protocol of this study was approved by the Institutional Human Research Committee of Tianjin Chest Hospital Ethics Committee (approval no. 2021LW-004). This study was a retrospective review of medical records; therefore, the need for informed consent was waived.
2.2. Diagnostic Criteria for CI-AKI
CI-AKI was defined as a ≥25% or ≥44.2 µmol/L increase in serum creatinine levels (compared with the baseline value) within 24–72 h of contrast medium administration [12]. This definition allowed the exclusion of patients with renal impairment induced by other causes.
2.3. Treatment Groups
First, the patients were assigned to groups according to whether or not the PCSK9 inhibitor evolocumab was administered before the intervention. There were 149 patients in the evolocumab group and 160 in the control group. The first group received 140 mg of evolocumab subcutaneously on admission to the emergency department. The control group did not receive evolocumab. The included patients were divided into 42 cases in the CI-AKI group and 267 cases in the non-CI-AKI group, according to the occurrence of CI-AKI postoperatively.
All patients were preoperatively treated with aspirin 300 mg, ticagrelor 180 mg or clopidogrel 600 mg, and atorvastatin 20 mg or rosuvastatin 10 mg. During the procedure, unfractionated heparin (80–100 U/kg) or bivalirudin (continued until 4 h after PCI) was administered. Furthermore, glycoprotein IIb/IIIa receptor antagonists, nicorandil, or sodium nitroprusside, were administered as indicated by the intraprocedural situation (slow flow or no-reflow). Most patients received the nonionic contrast agent iodixanol, while others received iopromide. Experienced interventional physicians in the Chest Pain Center at Tianjin Chest Hospital performed all PCI procedures. After PCI, all patients received standard medications for coronary artery disease, such as renin-angiotensin-aldosterone system (RAAS) inhibitors and beta-blockers, according to the guideline on the management of blood cholesterol, guideline for PCI, and guideline for the management of ST-elevation myocardial infarction, in addition to antiplatelet [13, 14], anticoagulation, and lipid-lowering therapies. If a patient with diabetes received metformin before the intervention, the administration of the drug was terminated 48 h postoperatively, and drug use was resumed only after a normal renal function review 48 h later. During this period, insulin was administered to control blood glucose levels as appropriate.
2.4. Baseline and Clinical Information on the Patients
Patient data recorded on admission included sex, age, body weight, previous myocardial infarction, history of hypertension and diabetes mellitus, and use of RAAS inhibitors, statins, or evolocumab.
2.5. Laboratory Parameters
2.5.1. Blood Biochemistry
Serum creatinine, high-sensitivity C-reactive protein, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (LDL-C) levels were measured using the Cobas c701 (Roche Diagnostics International AG, Rotkreuz, Switzerland) automatic biochemistry analyzer. Blood was collected from the cubital vein in all patients. Baseline serum creatinine levels and other indices were measured in patients after admission or as emergency checks before PCI. Serum creatinine indices were collected 24–72 h after the procedure. The remaining measurements were collected from patients after they fasted for 8 h the next morning.
2.5.2. Cardiac Markers
Troponin T (TNT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were measured using the Cobas e602 fully automated immunoassay analyzer (Roche Diagnostics International AG, Rotkreuz, Switzerland). Blood was collected from the cubital vein in all patients. Baseline TNT and NT-proBNP levels and other indices were measured in patients after admission or as emergency checks before PCI. The remaining measurements were collected from patients after they fasted for 8 h the next morning.
2.5.3. Routine Blood Tests
The automated XN-9000 hematology analyzer (Sysmex Corporation, Kobe, Japan) was used for routine blood tests. Blood was collected from the cubital vein in all patients. Baseline routine blood tests were performed in patients after admission or as emergency checks before the intervention. The remaining measurements were collected from patients after they fasted for 8 h the next morning.
2.6. Echocardiography Indicators
All patients underwent examination with the CX50 cardiac ultrasound machine (Philips, Amsterdam, Netherlands).
2.7. Clinical Indicators
Clinical indicators included systolic and diastolic blood pressures taken on the day of the PCI, the intake of fluid during the first day after PCI, and the dose of the contrast agent.
2.8. Statistical Methods
Data analyses were performed using SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). First, patients were assigned to groups according to whether or not the PCSK9 inhibitor evolocumab was administered before the intervention, and the change rate in serum creatinine level was compared between the two groups before and after the intervention. Second, the included patients were grouped according to whether or not they had CI-AKI after the intervention, and the suspected factors affecting CI-AKI were screened out. Continuous variables that conformed to a normal distribution were expressed as means and SDs, and the t-test was used to analyze such variables. Continuous variables that did not conform to the normal distribution were expressed as medians (P25–P75) and were analyzed using nonparametric tests.
Count data were expressed as percentages (%) and compared using the χ2 test. Logistic regression analysis was performed to assess whether the administration of evolocumab (independent variable) affected the occurrence of CI-AKI (dependent variable). According to the risk score for the prediction of CI-AKI [15], the model was adjusted for the following confounders: advanced age (>70 years), previous myocardial infarction, diabetes, hypertension, anemia (hemoglobin (Hb) level <110 g/L), contrast agent dose >150 mL, left ventricular ejection fraction (LVEF) <45%, emergency PCI, and suspicious influencing factors screened out by univariate analysis. A p value of <0.05 indicated statistical significance.
3. Results
3.1. Comparison of Baseline Data between the Evolocumab and Control Groups
The age of the patients included in this study varied from 27 to 90 (63.35, SD: 10.62) years, and the percentage of male patients was 71.84%. The percentage of iodixanol use was significantly lower in the evolocumab group than in the control group (p < 0.05). No significant difference was observed regarding all other parameters between the groups (p > 0.05) (Table 1).
Table 1.
Comparison of baseline data between those who received evolocumab and controls.
| Control group (n = 160) | Evolocumab group (n = 149) | χ 2/t/Z | p value | |
|---|---|---|---|---|
| Male sex, n (%) | 120 (75.0) | 102 (68.46) | 1.63 | 0.20 |
| Age (years), mean, SD | 64.15, 10.73 | 62.49, 10.45 | 1.38 | 0.17 |
| STEMI, n (%) | 103 (64.38) | 99 (66.44) | 0.15 | 0.70 |
| Old MI, n (%) | 28 (17.50) | 26 (17.45) | <0.01 | 0.99 |
| Diabetes mellitus, n (%) | 58 (36.25) | 57 (38.26) | 0.13 | 0.72 |
| Hypertension, n (%) | 88 (55.00) | 96 (64.43) | 2.85 | 0.09 |
| SBP (mmHg), median (IQR) | 134.00 (123.00–145.00) | 134.00 (123.00–145.00) | −0.14 | 0.89 |
| DBP (mmHg), median (IQR) | 78.00 (70.00–89.00) | 78.00 (70.00–88.75) | −0.60 | 0.55 |
| Atorvastatin, n (%) | 91 (56.88) | 80 (53.69) | 0.32 | 0.57 |
| RAAS inhibitors, n (%) | 143 (89.38) | 126 (84.56) | 1.59 | 0.21 |
| Killip class I, n (%) | 157 (98.13) | 141 (94.63) | 2.74 | 0.10 |
| LVEF (%), median (IQR) | 50.00 (45.00–56.00) | 51.50 (46.00–56.00) | −0.87 | 0.39 |
| NT-pro-BNP (ng/L), median (IQR) | 684.00 (206.20–1582.00) | 719.60 (328.65–1510.00) | −0.57 | 0.57 |
| TNT (ng/mL), median (IQR) | 1.74 (0.64–3.39) | 1.43 (0.43–3.11) | −1.43 | 0.15 |
| Hb (g/L), median (IQR) | 133.00 (125.00–145.00) | 134.00 (122.25–145.00) | −0.01 | 0.99 |
| Hs-CRP (mg/L), median (IQR) | 6.34 (2.25–23.10) | 5.01 (2.13–13.67) | −1.16 | 0.25 |
| HDL-C (mmol/L), median (IQR) | 1.03 (0.87–1.24) | 1.00 (0.83–1.20) | −1.11 | 0.27 |
| LDL-C (mmol/L), median (IQR) | 3.17 (2.51–3.87) | 3.23 (2.43–4.24) | −0.02 | 0.99 |
| Contrast agent (mL), median (IQR) | 140.00 (130.00–150.00) | 140.00 (120.00–150.00) | −0.73 | 0.47 |
| Emergency PCI, n (%) | 103 (64.38) | 99 (66.44) | 0.15 | 0.70 |
| Preoperative hydration, n (%) | 43 (26.88) | 29 (19.46) | 2.37 | 0.12 |
| Postoperative hydration, n (%) | 157 (98.13) | 147 (98.66) | 0.14 | 0.71 |
| Iodixanol, n (%) | 154 (96.25) | 118 (79.19) | 21.29 | <0.01 |
χ 2/t/Z, a test used to compare parameters; SD, standard deviation; STEMI, acute ST-segment elevation myocardial infarction; MI, myocardial infarction; SBP, systolic blood pressure; IQR, interquartile range; DBP, diastolic blood pressure; RAAS, renin-angiotensin-aldosterone system; LVEF, left ventricular ejection fraction; NT-pro-BNP, N-terminal probrain natriuretic peptide; TNT, troponin T; Hb, hemoglobin; hs-CRP, high-sensitivity C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCI, percutaneous coronary intervention.
3.2. Comparison of CI-AKI Indicators between the Evolocumab and Control Groups
The difference in the post-PCI serum creatinine levels between the evolocumab and control groups was significant (Z = −3.69, p < 0.01), and the creatinine level in the control group was higher than that in the evolocumab group. The changes in serum creatinine levels from pre-PCI to post-PCI were significantly different between the two groups (Z = −3.28, p < 0.01). The prevalence of CI-AKI was significantly higher (χ2 = 11.6, p < 0.01) in the control group than in the evolocumab group (Table 2).We further evaluated the correlation between exposure factor and outcome. The relative risk(RR) between the use of evolocumab and the occurrence of CI-AKI was 0.34(95% CI 0.17-0.66,p<0.01).This result indicate a significant association between the use of evolocumab and a reduction in the incidence of CI-AKI.
Table 2.
Comparison of pre- and postintervention kidney function and contrast-induced acute renal injury between those who received evolocumab and controls.
| Control group (n = 160) | Evolocumab group (n = 149) | χ 2/t/Z | p value | |
|---|---|---|---|---|
| Pre-PCI Cr (μmol/L), median (IQR) | 78.00 (67.00–89.00) | 78.00 (65.00–87.5) | −0.96 | 0.34 |
| Post-PCI Cr (μmol/L), median (IQR) | 86.00 (73.00–99.00) | 80.00 (69.00–90.00) | −3.69 | <0.01 |
| Cr change pre- to post-PCI (%), median (IQR) | 7.14 (1.12–20.26) | 3.45 (−1.18–12.79) | −3.28 | <0.01 |
| CI-AKI, n (%) | 32 (20.00) | 10 (6.70) | 11.60 | <0.01 |
χ 2/t/Z, a test used to compare parameters. IQR, interquartile range; PCI, percutaneous coronary intervention; Cr, creatinine; CI-AKI, contrast-induced acute renal injury.
3.3. Comparison of Baseline Data between the CI-AKI and Non-CI-AKI Groups
On re-examination of serum creatinine levels, 42 of the 309 patients met the diagnostic criteria of CI-AKI, and the incidence of CI-AKI was 13.59%. Univariate analysis revealed significant differences in diabetes history, Killip class I, and use of evolocumab (all p < 0.05) between the CI-AKI and non-CI-AKI groups (Table 3).
Table 3.
Comparison of baseline data between the CI-AKI and non-CI-AKI groups.
| Non-CI-AKI group (n = 267) | CI-AKI group (n = 42) | χ 2/t/Z | p value | |
|---|---|---|---|---|
| Male sex, n (%) | 194 (72.66) | 28 (66.67) | 0.64 | 0.42 |
| Age (years), mean, SD | 63.07, 10.78 | 65.14, 9.45 | −1.18 | 0.24 |
| STEMI, n (%) | 173 (64.79) | 29 (69.05) | 0.29 | 0.59 |
| Old MI, n (%) | 45 (16.85) | 9 (21.43) | 0.53 | 0.47 |
| Diabetes mellitus, n (%) | 90 (50.85) | 25 (59.52) | 10.35 | <0.01 |
| Hypertension, n (%) | 154 (57.68) | 30 (71.43) | 2.85 | 0.09 |
| SBP (mmHg), median (IQR) | 134.00 (123.00–145.00) | 135.00 (122.50–150.50) | −1.00 | 0.32 |
| DBP (mmHg), median (IQR) | 78.00 (70.00–86.00) | 80.00 (69.75–90.50) | −0.73 | 0.47 |
| Evolocumab, n (%) | 139 (52.06) | 10 (23.81) | 11.60 | <0.01 |
| Atorvastatin, n (%) | 147 (55.06) | 24 (57.14) | 0.06 | 0.80 |
| RAAS inhibitors, n (%) | 229 (85.77) | 40 (95.24) | 2.89 | 0.09 |
| Killip class I, n (%) | 260 (97.38) | 38 (90.48) | 5.04 | 0.03 |
| LVEF (%), median (IQR) | 51.00 (46.00–56.00) | 50.00 (42.00–56.25) | −1.11 | 0.27 |
| NT-pro-BNP (ng/L), median (IQR) | 679.00 (212.50–1475.00) | 852.50 (428.00–2489.00) | −1.56 | 0.12 |
| TNT (ng/mL), median (IQR) | 1.59 (0.54–3.19) | 1.50 (0.55–6.67) | −0.87 | 0.39 |
| Hb (g/L), median (IQR) | 133.00 (123.00–144.00) | 137.00 (126.00–148.00) | −1.30 | 0.20 |
| Hs-CRP (mg/L), median (IQR) | 5.19 (2.10–18.60) | 9.35 (2.64–15.63) | −1.18 | 0.24 |
| HDL-C (mmol/L), median (IQR) | 1.01 (0.86–1.23) | 1.05 (0.82–1.35) | −0.56 | 0.58 |
| LDL-C (mmol/L), median (IQR) | 3.17 (2.46–4.21) | 3.35 (2.57–3.75) | −0.54 | 0.59 |
| Contrast agent (mL), median (IQR) | 140.00 (130.00–150.00) | 140.00 (130.00–150.00) | 0.65 | 0.52 |
| Emergency PCI, n (%) | 171 (64.04) | 31 (73.81) | 1.53 | 0.22 |
| Preoperative hydration, n (%) | 60 (28.99) | 12 (28.57) | 0.76 | 0.39 |
| Postoperative hydration, n (%) | 263 (98.50) | 41 (97.62) | 0.18 | 0.67 |
| Iodixanol, n (%) | 233 (87.27) | 39 (92.86) | 1.08 | 0.30 |
χ 2/t/Z, a test used to compare parameters. CI-AKI, contrast-induced acute renal injury; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction; MI, myocardial infarction; SBP, systolic blood pressure; IQR, interquartile range; DBP, diastolic blood pressure; RAAS, renin-angiotensin-aldosterone system; LVEF, left ventricular ejection fraction; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; TNT, troponin T; Hb, hemoglobin; hs-CRP, high-sensitivity C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCI, percutaneous coronary intervention.
3.4. Association between Evolocumab and CI-AKI Prevalence
Three suspected risk factors for CI-AKI identified using univariate analysis were diabetes mellitus, evolocumab use, and Killip class I. Regression analysis was performed on these three factors and other clinically recognized factors affecting CI-AKI occurrence [15], including advanced age (>70 years), previous myocardial infarction, diabetes, hypertension, anemia (Hb level <110 g/L), contrast agent dose >150 mL, LVEF <45%, and emergency PCI. The results showed that the use of evolocumab was a protective factor for CI-AKI occurrence (odds ratio (OR) = 0.28, 95% CI 0.11–0.71, p < 0.01), whereas diabetes mellitus and LVEF <45% were risk factors for CI-AKI (Table 4, Figure 1).
Table 4.
Logistic regression analysis of patients with acute myocardial infarction undergoing coronary intervention (n = 309).
| B | SE | Wald c2 | p value | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Evolocumab | 1.62 | 0.46 | 12.65 | <0.01 | 5.07 | 2.07–12.39 |
| Killip class I | −1.26 | 0.78 | 2.59 | 0.11 | 0.29 | 0.06–1.31 |
| Hs-CRP | <0.01 | 0.01 | <0.01 | 0.99 | 1.00 | 0.99–1.02 |
| Emergency PCI | 0.31 | 0.44 | 0.51 | 0.47 | 1.37 | 0.58–3.22 |
| Age >70 years | -0.55 | 0.42 | 1.76 | 0.19 | 0.58 | 0.26–1.30 |
| Hypertension | −0.23 | 0.44 | 0.28 | 0.60 | 0.80 | 0.34–1.85 |
| LVEF <45% | −0.58 | 0.42 | 1.87 | 0.17 | 0.56 | 0.25–1.28 |
| Anemia | −0.09 | 0.87 | 0.01 | 0.92 | 0.92 | 0.17–4.99 |
| Diabetes mellitus | −1.09 | 0.40 | 7.50 | <0.01 | 0.34 | 0.15–0.73 |
| Previous MI | 0.10 | 0.48 | 0.04 | 0.84 | 1.10 | 0.43–2.80 |
| Contrast medium dose >150 mL | −0.44 | 0.51 | 0.76 | 0.39 | 0.64 | 0.24–1.74 |
| Iodixanol | −0.13 | 0.73 | 0.03 | 0.86 | 0.88 | 0.21–3.70 |
B, beta; SE, standard error; OR, odds ratio; CI, confidence interval; hs-CRP, high-sensitivity C-reactive protein; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Figure 1.
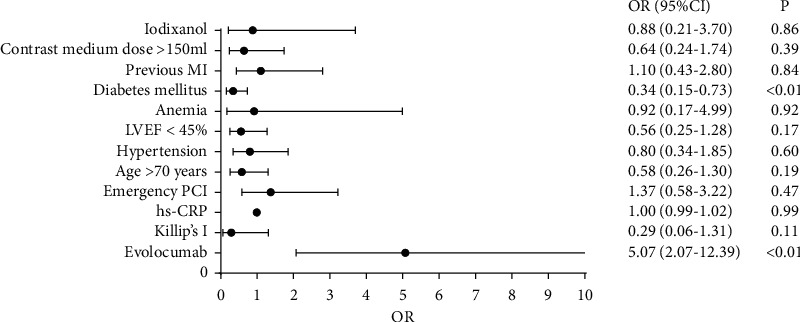
Logistic regression analysis of patients with acute myocardial infarction undergoing coronary intervention (n = 309). OR, odds ratio; CI, confidence interval; hs-CRP, high-sensitivity C-reactive protein; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
4. Discussion
This retrospective study investigated the prevalence of CI-AKI after PCI, the preferred treatment for acute myocardial infarction. Our results show that patients who received evolocumab, a PCSK9 inhibitor, were significantly less likely to have CI-AKI than those who did not receive evolocumab.
The prevention and treatment of iatrogenic complications have become important topics in healthcare. The currently used CI-AKI prevention measures included reducing the dose of contrast agents, using isotonic or hypotonic nonionic contrast agents, and sufficiently hydrating patients.
In this study, the proportion of patients who received isotonic contrast agent (iodixanol) in the control and evolocumab groups was 96.25% and 79.19%, respectively (p < 0.01), grouped according to the presence or absence of evolocumab. The proportion of patients who received iodixanol in the non-CI-AKI and CI-AKI groups was 87.27% and 92.86%, respectively (p=0.30), grouped according to the presence or absence of CI-AKI. The proportion of patients who received hypotonic contrast in the control and evolocumab group was 3.75% and 20.81%, respectively. Theoretically, the incidence of CI-AKI should be higher in the evolocumab group than in the control group. However, the actual results showed that the opposite was true, such that the incidence of CI-AKI was lower in the evolocumab group, suggesting that PCSK9 inhibitors protected against CI-AKI. Therefore, we believe that the application of PCSK9 inhibitors may be a protective factor in the development of CI-AKI.
Hydration therapy administered orally or intravenously is currently recognized as the most effective means of CI-AKI prevention. Hydration during the peri-PCI period dilutes the contrast agent as it enters the body, reducing the effects of its osmotic pressure and concentration on the kidneys. However, hydration therapy cannot prevent CI-AKI in high-risk patients, and approximately 11% of patients with chronic renal dysfunction develop CI-AKI despite adequate hydration therapy [2].
Recently, the role of statins in preventing CI-AKI has been investigated more extensively. Statins improve endothelial function, inhibit inflammation and apoptosis, and exert antioxidant and antithrombotic effects [16]. A retrospective analysis by Khanal et al. showed that statin administration before PCI significantly reduced CI-AKI incidence [17]. However, less than one-third of Chinese patients who receive statin therapy comply with medication adherence, especially those with very high risks of atherosclerotic cardiovascular disease [18]. Simply optimizing statin therapy cannot meet the demand for lipid reduction, and more intensive therapy is needed. The American Society of Nephrology guidelines emphasize stricter blood lipid management for patients with cardiovascular and chronic kidney diseases [19]. The addition of evolocumab to statin therapy has further reduced LDL-C levels by 59–75% [3, 4, 20]. The EVOPACS study showed that evolocumab administration within 1–3 days of the commencement of an ACS allowed >95% of patients to achieve an LDL-C level of <1.8 mmol/L [5].
In pre-PCI statin and hydration therapy, the results showed that the prevalence of CI-AKI in patients with acute myocardial infarction who received evolocumab was 6.7%, which was significantly less than the rate of 20.0% in the group that did not receive the PCSK9 inhibitor. Logistic regression analysis confirmed that the PCSK9 inhibitor was a protective factor against CI-AKI in patients undergoing PCI. Therefore, this study explored whether PCSK9 inhibitors reduce the prevalence of CI-AKI in patients undergoing PCI.
The mechanism underlying this reduction in CI-AKI prevalence after PCI in patients receiving evolocumab is not entirely clear, although there are a few potential explanations. A meta-analysis concluded that PCSK9 inhibitors inhibit pyroptosis independent of their lipid-lowering effects [6]. A study showed that PCSK9 regulates pyroptosis through mitochondrial DNA damage in mice with chronic myocardial ischemia [21]. Pyroptosis is a form of programmed cell death observed in many types of cells, including immune cells, vascular smooth muscle cells, cardiomyocytes, and renal tubular epithelial cells [22]. PCSK9 was closely associated with macrophage pyroptosis and the progression of inflammation in atherosclerosis. Caspase-11-mediated tubular epithelial pyroptosis has been confirmed as the basis of CI-AKI [23]. Consequently, reducing pyroptosis may diminish the degree of contrast-induced tubular epithelial injury.
Some studies have shown that reduced PCSK9 transcription is associated with increased expression of the apolipoprotein E2 receptor. Cells lacking this receptor gene lose their PCSK9-dependent apoptosis ability, whereas the inhibition of PCSK9 expression may also reduce apoptosis [7, 8]. Contrast-induced apoptosis in vascular endothelial and tubular epithelial cells promotes CI-AKI development [10, 11]. Based on these findings, it is hypothesized that the administration of PCSK9 inhibitors may reduce CI-AKI by decreasing pyroptosis and apoptosis.
With the growing evidence on the effects of PCSK9 inhibitors, their importance in treatment guidelines has increased significantly. The Fourier and OSLER-1 trials and EVOPACS study recommend PCSK9 inhibitors for treating atherosclerotic cardiovascular disease [3–5]. PCSK9 inhibitors are a new type of lipid-lowering drug with a more robust lipid-lowering effect and higher safety profile.
The results of this study should be interpreted within its limitations. First, this was a nonrandomized retrospective study whose inherent weakness cannot be avoided. Second, this study was conducted in a single center; therefore, it may be weaker in methodology than studies using multicenter sampled populations. Third, the effects of many drugs have not been ruled out, especially some renal protective drugs.
5. Conclusions
This study shows that PCSK9 inhibitor therapy combined with hydration and statin administration is a promising method to prevent CI-AKI. The sample size of this single-center clinical retrospective study was limited because of which it was impossible to exclude the influence of confounding factors completely. Moreover, this study only investigated the incidence of CI-AKI in patients with acute myocardial infarction; the effect of PCSK9 inhibitors on CI-AKI incidence in other ASCVD (Atherosclerotic Cardiovascular Disease) patients undergoing PCI remains to be prospectively studied.
Acknowledgments
The authors have benefited from the Tianjin Institute of Cardiovascular Diseases and Department of Emergency Medicine staff, who assisted with data collection and contributed to many invaluable suggestions. Therefore, the authors thank these individuals for their kind help. The authors also thank Editage (https://www.editage.com) for English language editing. This study was funded by the Health Science and Technology Project of Tian Jin city (grant number: TJWJ2021QN056) and Tianjin Key Medical Discipline (Specialty) Construction Project (grant number: TJYXZDXK-055B).
Contributor Information
Hongliang Cong, Email: hongliangcong@126.com.
Ximing Li, Email: ljsunlight@126.com.
Data Availability
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Meier P., Ko D. T., Tamura A., Tamhane U., Gurm H. S. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Medicine . 2009;7 doi: 10.1186/1741-7015-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikolsky E., Mehran R. Understanding the consequences of contrast-induced nephropathy. Reviews in Cardiovascular Medicine . 2003;4(Suppl 5):S10–S18. [PubMed] [Google Scholar]
- 3.Sabatine M. S., De Ferrari G. M., Giugliano R. P., et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease: analysis from fourier. Circulation . 2018;138(8):756–766. doi: 10.1161/circulationaha.118.034309. [DOI] [PubMed] [Google Scholar]
- 4.Koren M. J., Sabatine M. S., Giugliano R. P., et al. Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. Journal of the American College of Cardiology . 2019;74(17):2132–2146. doi: 10.1016/j.jacc.2019.08.1024. [DOI] [PubMed] [Google Scholar]
- 5.Koskinas K. C., Windecker S., Pedrazzini G., et al. Evolocumab for early reduction of LDL cholesterol levels in patients with acute coronary syndromes (EVOPACS) Journal of the American College of Cardiology . 2019;74(20):2452–2462. doi: 10.1016/j.jacc.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y. X., Li S., Liu H. H., Li J. J. Impact of PCSK9 monoclonal antibodies on circulating hs-CRP levels: a systematic review and meta-analysis of randomised controlled trials. BMJ Open . 2018;8(9) doi: 10.1136/bmjopen-2018-022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C. Y., Tang Z. H., Jiang L., Li X. F., Jiang Z. S., Liu L. S. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Molecular and Cellular Biochemistry . 2012;359(1-2):347–358. doi: 10.1007/s11010-011-1028-6. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q., Tang Z. H., Peng J., et al. The dual behavior of PCSK9 in the regulation of apoptosis is crucial in Alzheimer’s disease progression (Review) Biomedical Reports . 2014;2(2):167–171. doi: 10.3892/br.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F., Lu J., Yang X., et al. Acetylbritannilactone attenuates contrast-induced acute kidney injury through its anti-pyroptosis effects. Bioscience Reports . 2020;40(2) doi: 10.1042/bsr20193253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J., Wu G., Yang C., Li Y., Jing Q., Han Y. Rosuvastatin attenuates contrast-induced nephropathy through modulation of nitric oxide, inflammatory responses, oxidative stress and apoptosis in diabetic male rats. Journal of Translational Medicine . 2015;13(1) doi: 10.1186/s12967-015-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X., Yang J., Li L., et al. Atorvastatin protects against contrast-induced nephropathy via anti-apoptosis by the upregulation of Hsp27 in vivo and in vitro. Molecular Medicine Reports . 1972;15(4):p. 1963. doi: 10.3892/mmr.2017.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schilp J., de Blok C., Langelaan M., Spreeuwenberg P., Wagner C. Guideline adherence for identification and hydration of high-risk hospital patients for contrast-induced nephropathy. BMC Nephrology . 2014;15(1):p. 2. doi: 10.1186/1471-2369-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy S. M., Stone N. J., Bailey A. L. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Journal of the American College of Cardiology . 2018;139(25) [Google Scholar]
- 14.Levine G. N., Bates E. R., Blankenship J. C., et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. Journal of the American College of Cardiology . 2016;67(10):1235–1250. doi: 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Fu N., Li X., Yang S., et al. Risk score for the prediction of contrast-induced nephropathy in elderly patients undergoing percutaneous coronary intervention. Angiology . 2013;64(3):188–194. doi: 10.1177/0003319712467224. [DOI] [PubMed] [Google Scholar]
- 16.Kostapanos M. S., Liberopoulos E. N., Elisaf M. S. Statin pleiotropy against renal injury. Journal of the CardioMetabolic Syndrome . 2009;4(1) doi: 10.1111/j.1559-4572.2008.00052.x. [DOI] [PubMed] [Google Scholar]
- 17.Khanal S., Attallah N., Smith D. E., et al. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. The American Journal of Medicine . 2005;118(8):843–849. doi: 10.1016/j.amjmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Gitt A. K., Lautsch D., Ferrieres J., et al. Contemporary data on low-density lipoprotein cholesterol target value attainment and distance to target in a cohort of 57, 885 statin-treated patients by country and region across the world. Data in Brief . 2016;9:616–620. doi: 10.1016/j.dib.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease Outcomes Quality Initiative (K/DOQI) Group. Kidney Disease Outcomes Quality Initiative (K/DOQI) Group, “K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney disease”. American Journal of Kidney Diseases . 2003;41(4 Suppl 3) [PubMed] [Google Scholar]
- 20.Robinson J. G., Nedergaard B. S., Rogers W. J., et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA . 2014;311(18):1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Li X., Liu S., et al. PCSK9 regulates pyroptosis via mtDNA damage in chronic myocardial ischemia. Basic Research in Cardiology . 2020;115(6):p. 66. doi: 10.1007/s00395-020-00832-w. [DOI] [PubMed] [Google Scholar]
- 22.Kong F., Ye B., Cao J., et al. Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB and P2X7R signaling in PMA-induced macrophages. Frontiers in Pharmacology . 2016;7:p. 369. doi: 10.3389/fphar.2016.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z., Shao X., Jiang N., et al. Caspase-11-mediated tubular epithelial pyroptosis underlies contrast-induced acute kidney injury. Cell Death and Disease . 2018;9(10):p. 983. doi: 10.1038/s41419-018-1023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author on reasonable request.


