Abstract
Background
Prenatal alcohol exposure can lead to a wide range of neurological and behavioral deficits, including alterations in motor domains. However, much less is known about the effects of prenatal cannabis exposure on motor development, despite cannabis being the most consumed illicit drug among women. Cannabis use among pregnant women has become increasingly popular given the widespread perception that consumption is safe during pregnancy. Moreover, alcohol and cannabis are commonly used together, even among pregnant women. Yet few studies have explored the potential consequences of combined prenatal exposure on behavioral domains.
Methods
Using our previously established model, during gestational days 5 to 20, four groups of pregnant Sprague–Dawley rats were exposed to vaporized alcohol, delta‐9‐Tetrahydrocannabinol (THC) via electronic (e‐) cigarettes, the combination of alcohol and THC, or a vehicle. Following birth, offspring were tested on early sensorimotor development, adolescent motor coordination, and adolescent activity levels.
Results
Prenatal THC e‐cigarette exposure delayed sensorimotor development early in life and impaired motor coordination later in early adolescence; combined prenatal alcohol and THC exposure did not have additive effects on sensorimotor development. However, combined prenatal exposure produced hyperactivity among male offspring.
Conclusions
Prenatal cannabis exposure may lead to impaired motor skills throughout early development and combined exposure with alcohol during gestation may lead to hyperactivity in early adolescence. These findings have important implications for informing pregnant women of the risks to the fetus associated with prenatal cannabis exposure, with and without alcohol, and could influence public policy.
Keywords: alcohol, e‐cigarette, motor, prenatal, THC
Pregnant Sprague‐Dawley rats were exposed daily to alcohol, THC via e‐cigarettes, the combination, or a vehicle. Following birth, offspring were examined for early sensorimotor development, adolescent motor coordination, and adolescent activity levels. Prenatal THC exposure delayed sensorimotor development and coordination early in life, whereas combined prenatal exposure to alcohol and THC lead to adolescent hyperactivity. These findings have important implications for pregnant women regarding the risks associated with prenatal cannabis exposure, with and without alcohol, and influencing related public policy.
![]()
INTRODUCTION
It is well known that prenatal alcohol exposure can lead to a wide range of physical and behavioral alterations, known as fetal alcohol spectrum disorders (FASD). However, alcohol is not the only drug consumed by women during pregnancy. Cannabis is the most common illicit drug consumed by pregnant women; prevalence rates of cannabis use during pregnancy are estimated to be between 3% and 10% in the United States, with some variability dependent on the legal status of cannabis across states (Volkow et al., 2019). In fact, some women purposefully use cannabis while pregnant to combat symptoms such as nausea and pain, although recent reports suggest that cannabis use can actually provoke nausea and vomiting (Kim et al., 2018). These use rates and patterns are undoubtedly fueled by the perception that cannabis use is safe during pregnancy (Jarlenski et al., 2017).
Despite the high rates of use, the potential consequences of prenatal cannabis exposure on the developing fetus are not well understood, as results from both clinical and preclinical studies have been mixed. Results vary greatly based on developmental timing of exposure, the type and/or dose of cannabinoid, the type of control groups used, and the age and domain of behavior testing (Huizink, 2014; Schneider, 2009). Variability is likely to be even more enhanced given the wider variety in concentrations of delta‐9‐tetrahydrocannabinol (THC), the primary psychoactive component. Clinically, the long‐term consequences of the various cannabis products on the market today will not be known for years to come.
In addition to changes in potency and legal status, the routes of administration for cannabis use have drastically changed. The use of electronic cigarettes (e‐cigarettes) for cannabis consumption has greatly increased in popularity; estimated rates of e‐cigarette use among pregnant women are 5% to 14% (Cardenas et al., 2019), which includes THC vaping. This high rate is driven by the assumption that using e‐cigarettes is safer than traditional smoking (Mark et al., 2015). However, consuming cannabis via e‐cigarettes leads to higher drug and metabolite levels in the blood in both the consumer and the fetus than traditional routes (Young‐Wolff et al., 2020). Thus, the potential consequences of prenatal cannabis exposure via vaping may be even more detrimental than other routes of administration. Medical professionals have requested research addressing whether prenatal e‐cigarette use leads to more severe health consequences (Suter et al., 2015), yet research is still in its infancy.
Moreover, polydrug consumption is also seen among pregnant women; for example, half of the women who report using cannabis during their pregnancy also report consuming alcohol (Hedden, 2015). Rates of alcohol and cannabis co‐consumption among pregnant women are likely underestimated, given that self‐report may be influenced by stigmatization and/or legal repercussions (Lange et al., 2014; Young‐Wolff et al., 2017). To date, few studies have investigated the effects of combined alcohol and cannabinoid exposure (Abel et al., 1987; Abel & Subramanian, 1990; Basavarajappa et al., 2008; Goldschmidt et al., 2004; Hansen et al., 2008; Nagre et al., 2015; Subbanna et al., 2013); thus, research in this area is desperately needed.
Prenatal cannabis and alcohol exposure may influence development in many behavioral domains, including motor function. Research on the effects of prenatal cannabinoid exposure on motor skill development and coordination has been mixed. On the one hand, prenatal THC exposure decreases early developmental motility (Fried, 1976), whereas early synthetic cannabinoid exposure may either impair motor coordination later in life (Shabani et al., 2011) or advance early motor development, but not affect motor coordination later in life (Breit et al., 2019a). Results from clinical research are similarly conflicting, leading to both impairments in motor skills (Richardson et al., 1995) and advanced motor behaviors (Fried & Watkinson, 1990).
Similarly, cannabis exposure during early development may also influence overall activity levels. Some preclinical studies indicate that developmental exposure to synthetic cannabinoids (Breit et al., 2019b; Mereu et al., 2003) and THC (Borgen et al., 1973; Navarro et al., 1994; Rubio et al., 1995) increases activity levels among rodents, while others find no change (Brake et al., 1987; Hutchings et al., 1989; Vardaris et al., 1976). Similar inconsistencies are seen in activity levels of children exposed to cannabis prenatally, reporting either hyperactivity (Fried et al., 1992; Goldschmidt et al., 2000) or no change in activity (Fried & Watkinson, 1988). Thus, it is relatively unclear how prenatal cannabis exposure alters motor‐related behaviors later in life, likely due to the methodological and other differences across these studies.
In contrast to the inconsistencies seen among the cannabis literature, it has been well established that prenatal alcohol exposure impairs motor skills. Individuals with FASD consistently show impaired fine and gross motor skills (Driscoll et al., 1990), and preclinical studies administering alcohol during early development show similar impairments in motor development and coordination (Driscoll et al., 1990; Idrus et al., 2017; Thomas et al., 2004, 2009). Moreover, developmental alcohol exposure is also often associated with attention deficit hyperactivity disorder symptoms present in clinical populations (O'malley & Nanson, 2002; Popova et al., 2016) and similarly leads to hyperactivity in rodents (Bond, 1981; Breit et al., 2019b; Ryan et al., 2008; Thomas et al., 2007).
Research examining the behavioral effects of combined alcohol and cannabinoid exposure during the gestational period is limited, despite the high prevalence of co‐consumption of alcohol and cannabis during pregnancy. The few studies that have explored alcohol and cannabinoid exposure during early development have primarily focused on the effects of either drug individually or combined effects on physical, physiological, or neural pathologies (Abel et al., 1987; Fish et al., 2019; Hansen et al., 2008). Our laboratory recently examined the effects of combined exposure to alcohol and cannabinoids during the third‐trimester equivalent. We illustrated that combined neonatal exposure to CP‐55,940 and alcohol impaired motor coordination and produced more severe hyperactivity, in part by reducing habituation (Breit et al., 2019a; Breit et al., 2019b). These results suggest that combined exposure to alcohol and THC during early development may have more severe effects on motor development, coordination, and activity than either drug alone. Moreover, combined exposure where THC is administered via e‐cigarette may be even more detrimental as it may alter drug metabolism in the blood of the user, as shown in both clinical (Downey et al., 2013; Hartman et al., 2015) and preclinical research (Breit et al., 2020).
The current study examined whether prenatal exposure to vaporized alcohol, THC via e‐cigarette, or the combination would alter sensorimotor development, motor coordination, and/or activity levels among offspring. We used a novel vapor inhalation model using commercially available e‐cigarette exposure, which intoxicates dams with alcohol and/or THC while avoiding confounding nutritional factors (Breit et al., 2020). Prenatal vaporized drug exposure occurred once daily during a period equivalent to the human first and second trimesters. The drug doses chosen for this paradigm produced a moderate binge dose of alcohol and a low–moderate dose of THC to mimic levels most commonly consumed in the general population (Andrenyak et al., 2017). Following birth, offspring were tested on a grip strength/hind limb coordination task early in development, a parallel bar motor coordination task, and open‐field activity in early adolescence to examine the potential effects of prenatal exposure on motor behaviors across development.
MATERIALS AND METHODS
Maternal vapor exposure
Dams
All procedures and behavioral tests included in this study were approved by the San Diego State University Institutional Animal Care and Use Committee and are in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals.
Detailed information regarding the dams used in this study has been previously published elsewhere (Breit et al., 2020). Naïve female Sprague–Dawley rats were obtained from Charles River Laboratories and acclimated for 2 weeks before any procedures began. Prior to breeding, intravenous catheters were surgically implanted to measure blood alcohol concentrations (BACs) and THC plasma levels during pregnancy. Following intravenous catheter placement, each dam was housed with a stud for up to 5 consecutive days. The presence of a seminal plug was deemed as gestational day (GD) 0; pregnant dams were then singly housed and monitored daily until the day of birth (typically GD 22). On GD 0, dams were also randomly assigned to 1 of 4 prenatal vapor inhalation exposure groups: the combination of ethanol (EtOH) and THC (EtOH + THC), EtOH alone (EtOH + Vehicle), THC alone (Air + THC), or controls (Air + Vehicle). Final n's of 10 to 13 dams completed the vapor inhalation paradigm for each exposure group (EtOH + THC: 10; EtOH + Vehicle: 12; Air + THC: 13; Air + Vehicle: 12).
Prenatal vapor inhalation
This model was designed to replicate co‐exposure to alcohol and THC e‐cigarette vapors in order to produce peak levels of each drug at similar time points. The vapor inhalation equipment (La Jolla Alcohol Research Inc) uses both heated flask vaporization of EtOH and e‐cigarette atomization for THC and Vehicle exposure. From GD 5 to 20, dams were exposed once daily to EtOH (68 ml/h; 95%, Sigma Aldrich) or Air via vapor inhalation (airflow of 10 L/min) for 3 h in a constant stream. Following EtOH or Air exposure, dams were then exposed to THC (100 mg/ml; NIDA Drug Supply Program) or the Vehicle (propylene glycol, Sigma Aldrich) via an e‐cigarette tank (SMOK V8 X‐Baby Q2) for 30 min (airflow of 2 L/min). E‐cigarette exposure to THC or the Vehicle was delivered in seven 6‐s puffs that were 5 min apart (30 min total), followed by 10 min of air exposure to clear out any residual vapors in the chambers. All doses and air flow rates were chosen to achieve desired drug levels in maternal blood, 150 mg/dl for alcohol and 20 ng/ml THC (Breit et al., 2020).
On PD 2, litters were culled to eight pups (four females and four males) to maintain consistency in pup growth, avoid food competition, and promote similar maternal behaviors across litters; this litter size is well‐supported within teratology research (Agnish & Keller, 1997; Chahoud & Paumgartten, 2009). To avoid potential litter effects, only one sex pair per litter (cohort 1) was tested on the early sensorimotor development and motor coordination tasks (Festing, 2006; Lazic & Essioux, 2013; Wainwright, 1998); a separate sex pair (cohort 2) was examined in open‐field activity chambers. Remaining offspring were used for other behavioral tasks (reported elsewhere). On PD 7, offspring were tattooed with nontoxic veterinary ink for identification purposes. Litters were weaned from the dam on PD 21 and separated by sex on PD 28. All motor testing took place between PD 12 and 34 (Figure 1). Subjects had ad libitum access to food and water, were weighed daily, and were acclimated to the testing room 30 min prior to each task.
FIGURE 1.
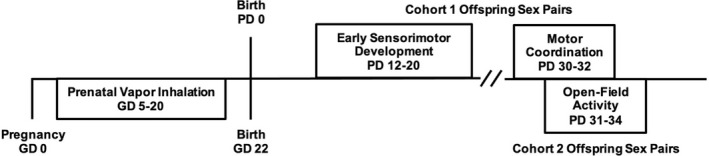
Timeline of the study design
Early sensorimotor development
Offspring completed a grip strength and hind limb coordination test daily from PD 12 to 20 to examine early sensorimotor development. Pups were placed with their forepaws on a wire (2‐mm diameter) suspended above a cage of bedding. Each subject was given two consecutive trials (30 s each) to either hold onto the wire for the full 30 s or place their hind limb on the wire; if either were achieved, the trial was recorded as successful. The first day of any success and the number of successes were recorded live by two investigators; all testing was also recorded for additional analysis.
Adolescent motor coordination
The same offspring were later tested for motor coordination and balance using a parallel bar task daily from PD 30 to 32. The apparatus included two parallel steel bars (0.5 cm diameter, 91 cm length) bolted between two platforms (15.5 cm × 17.8 cm) above a cage of bedding. The platforms have grooved slots (0.5 cm apart), allowing the width between the bars to be altered incrementally. The parallel bar testing was conducted in a room illuminated with a red light to promote locomotor activity.
Offspring were placed on one of the two platforms for 30 s to acclimate to the apparatus. Subjects were then placed in the middle of the parallel bars, with one forelimb and one hind limb on each bar. If subjects successfully traversed across the bars with four consecutive hind limb steps, the trial was deemed successful. If subjects' paws slipped, they fell, or they swung below the bars, the trial was considered a failure. With each successful trial, the width of the parallel bars was increased by 0.5 cm. Subjects were given up to 15 trials per day for 3 consecutive days. Testing ceased for the day once the maximum number of trials were reached (15), or if the subject failed on 5 consecutive trials. The trials to the first success, number of successful trials, maximum width achieved, and the success ratio (successful trials divided by total trials) were recorded each day.
Adolescent activity levels
Activity levels were examined using an open‐field activity chamber paradigm from PD 31 to 34; offspring tested in this domain were separate sex pairs from those tested on early sensorimotor development and motor coordination. Activity levels were assessed during the offspring's dark cycles (beginning at 18:00). Each subject was placed in an individual automated open‐field activity chamber with infrared beams (16 × 16 × 14 in; Hamilton‐Kinder) for 60 min; chambers were equipped with white noise and fans for ventilation. The number of infrared beam breaks, distance traveled (in), number of rears, entries into the center, and time spent in the center were recorded in 5‐min time bins for each day of testing.
Statistical analyses
Offspring motor data were analyzed using the Statistical Package for Social Sciences (IBM; version 27). All data were initially tested for normality (Shapiro–Wilk test). Data that were normally distributed were initially analyzed using a 2 (EtOH, Air) × 2 (THC, Vehicle) × 2 (Female, Male) design, with Student–Newman–Keuls (SNK) post hoc tests to identify individual group differences. Repeated measures (i.e., Day and Bin) were used to measure whether levels changed over days and/or time (body weight, parallel bar maximum width, and open‐field activity). Given the extensive literature regarding sex differences in teratology research (Terasaki et al., 2016; Weinberg et al., 2008), a priori planned comparisons between male and female offspring were always conducted. Binomial data, data with a limited range of outcomes, or data that were not normally distributed were analyzed nonparametrically using Mann–Whitney, Kruskal–Wallis, or Fishers‐exact analyses, where appropriate. Means (M) and standard error of the means (SEM) are reported when applicable. Significance levels for all paradigms were set at p < 0.05.
RESULTS
Body weights
Motor development
A total of 88 subjects completed both the early sensorimotor and the motor coordination tasks (EtOH + THC: 9 females, 8 males; EtOH + Vehicle: 13 females, 10 males; Air + THC: 13 females, 11 males; Air + Vehicle: 12 females, 12 males). Body weights for all tasks were analyzed with a EtOH × THC × Sex ANOVA with Day as a repeated measure. As previously published, neither litter size nor initial birth weights differed among any of the prenatal exposure groups (Breit et al., 2020).
During sensorimotor testing, subjects prenatally exposed to THC weighed less than those not exposed to THC, producing both a main effect of THC, F (1, 80) = 19.52, p < 0.001, and an interaction of THC × Day F (8, 640) = 7.85, p < 0.001. Although offspring in all groups gained significant weight during this developmental period, THC‐exposed offspring weighed less than Vehicle‐exposed offspring on each Day (p's < 0.001), and the THC‐related reductions were more pronounced as days progressed (Figure 2A). Thus, prenatal exposure to THC via e‐cigarettes reduced offspring body weights during this early period. In contrast, prenatal EtOH exposure did not alter body weights during this developmental period, nor were there any significant main or interactive effects of Sex (data not shown).
FIGURE 2.
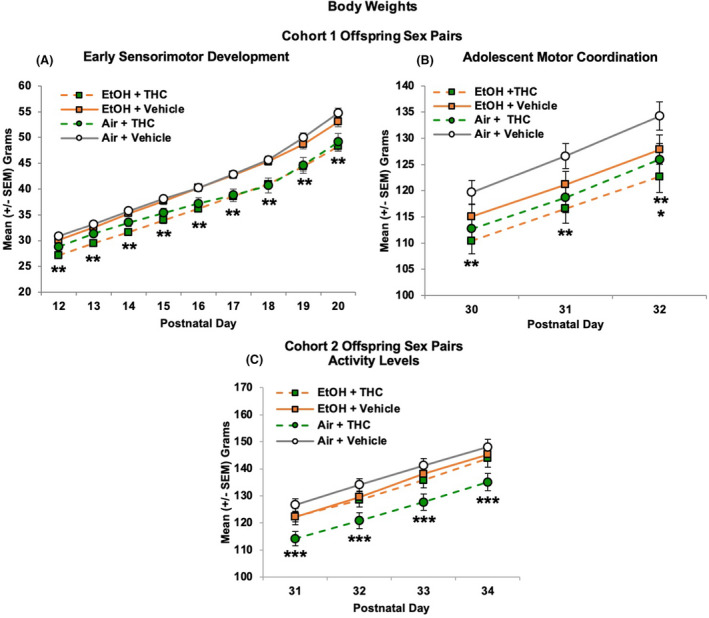
Offspring from Cohort 1 exposed to THC prenatally weighed significantly less throughout the early sensorimotor development paradigm (postnatal days 12 to 20; A) and the parallel bar paradigm (PD 30 to 32; B), alone or in combination with alcohol. Separately, there was an overall reduction in body weight of alcohol‐exposed subjects on PD 32 (B), an effect driven by the combined exposure group in Cohort 1. In Cohort 2, offspring exposed to THC alone weighed less than all other prenatal exposure groups during the open‐field activity testing (C). ** = THC (collapsed) < Vehicle (collapsed), p's < 0.05. * = EtOH (collapsed) < Air (collapsed), p = 0.05. *** = Air + THC < all other groups, p's < 0.05
Prenatal THC exposure continued to reduce body weight throughout parallel bar testing (PD 32). Offspring exposed to THC prenatally weighed less than their Vehicle‐exposed counterparts, alone or in combination with EtOH, as evident by a main effect of THC, F (1, 79) = 6.34, p < 0.05 (Figure 2B). In addition, a significant Day*EtOH interaction was observed, F (2, 158) = 3.43, p < 0.05. Offspring exposed to EtOH prenatally weighed less than their Air‐exposed counterparts on the last day of testing (PD 32; main effect of EtOH), F (1, 79) = 3.86, p = 0.05, although this effect was driven by the combined exposure group (Figure 2B), as body weights of subjects exposed to EtOH only did not significantly differ from that of controls. Overall, females weighed less than males (main effect of Sex: F1,79] = 30.29, p < 0.001) and gained weight more slowly than males over these days, leading to a Day*Sex interaction, F (2, 158) = 23.54, p < 0.001; however, Sex did not interact with EtOH or THC (data not shown).
Adolescent activity levels
Separate littermates (88 subjects) were examined for open‐field activity levels (EtOH + THC: 9 females, 9 males; EtOH + Vehicle: 12 females, 11 males; Air + THC: 13 females, 12 males; Air + Vehicle: 12 females, 12 males). Across Days, a main effect of Sex indicated that females weighed less than males, F (1, 82) = 47.24, p < 0.001, but Sex did not interact with prenatal exposure to EtOH or THC (data not shown). Overall, offspring exposed to THC only weighed significantly less than all other groups, producing significant interactions of EtOH*THC, F (1, 82) = 6.91, p < 0.05, Day*THC, F (3, 246) = 3.56, p < 0.05, and a main effect of THC, F (1, 82) = 10.87, p < 0.01. Subjects exposed to only THC weighed less than all other Groups on all days (p's < 0.05; Figure 2C). There were no statistically significant effects of EtOH on any day.
Early sensorimotor development
Shapiro–Wilk normality tests indicated that the behavioral data for this task were not normally distributed (p's < 0.05) due to the limited numerical range of outcomes, so nonparametric analyses were used. Separate Mann–Whitney U tests comparing main effects of EtOH and THC were initially conducted. In addition, since nonparametric analyses do not allow for examination of interactions, data were also analyzed across the 4 exposure groups using Kruskal–Wallis H tests. Lastly, for binomial data (i.e., able to perform or not), chi‐square comparisons were used. Females and males did not significantly differ on any behavioral outcome measures, so all data were collapsed by Sex.
Exposure to prenatal THC via e‐cigarettes significantly delayed the age of the first successful trial on the sensorimotor task (U = 648.00, p < 0.001; H = 9.85, p < 0.01; Figure 3A). In contrast, prenatal EtOH exposure had no significant effect on the first day of a successful trial (U = 870.00, p = 0.43). Chi‐square comparisons on each Day during the early sensorimotor task indicated that prenatal THC exposure delayed successful performance on this task, both alone and in combination with EtOH (Figure 3B). On PD 12, offspring exposed to the combination of EtOH + THC prenatally performed worse than those exposed to EtOH only (EtOH + Vehicle: X 2 = 3.91, p < 0.05) and controls (Air + Vehicle: X 2 = 8.13, p < 0.01), whereas THC‐exposed subjects (Air + THC) were impaired compared to control offspring (X 2 = 4.09, p < 0.01). Subjects exposed prenatally to the combination of EtOH + THC continued to perform worse than those exposed to EtOH only (X 2 = 4.37, p < 0.01) and controls (X 2 = 4.78, p < 0.01) on PD 13, but by PD 15, no differences were seen among groups, suggesting a catch‐up in sensorimotor development.
FIGURE 3.
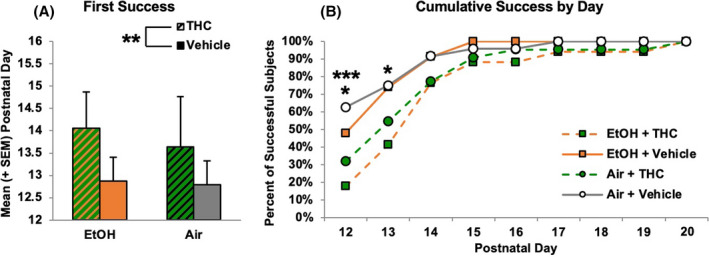
Prenatal exposure to THC via e‐cigarettes delayed early sensorimotor development. Although subjects exposed to any prenatal THC showed developmental delays (A), their motor development eventually caught up with that of controls (B). ** = THC (collapsed) > Vehicle (collapsed), p < 0.05. * = EtOH + THC < EtOH + Vehicle and Air + Vehicle, p's < 0.05. *** = Air + THC < Air + Vehicle, p < 0.05
Adolescent motor coordination
Prenatal THC exposure also significantly impaired performance on all measures of the parallel bar motor coordination task. A main effect of THC indicated that offspring exposed to THC prenatally required more trials to reach their first success, F (1, 68) = 7.24, p < 0.01 (Figure 4A) and had a lower success ratio on Day 1 compared with groups not exposed to THC, F (1, 68) = 10.38, p < 0.01 (Figure 4B), consistent with the percent of subjects successful on Day 1 (EtOH + THC: 41%, EtOH + Vehicle: 63%, Air + THC: 43%, Air + Vehicle: 65%). Prenatal alcohol exposure did not affect these outcomes or modify the effects of prenatal THC exposure.
FIGURE 4.
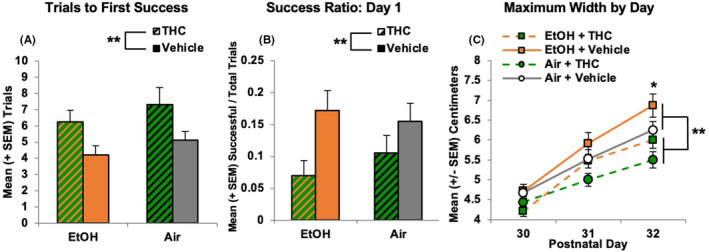
Prenatal THC exposure impaired motor coordination in adolescent subjects, increasing the number of trials before the first success (A), decreasing success ratios (B), and decreasing the maximum width achieved (C). ** = THC (collapsed) significantly different from Vehicle (collapsed) p's < 0.05. * = EtOH > EtOH + THC and Air + THC, p < 0.001
Finally, prenatal THC exposure also reduced the maximum width successfully traversed (main effect of THC), F (1, 68) = 12.17, p < 0.01 (Figure 4C). As expected, performance of subjects improved over days, F (2, 136) = 173.24, p < 0.001. However, there was also a significant interaction of Day*EtOH, F (2, 136) = 7.90, p < 0.001, as EtOH‐exposed offspring (collapsed across groups) were able to traverse greater widths compared to their Air‐exposed counterparts on the last day of testing (PD 32; main effect of EtOH), F (1, 68) = 5.14, p < 0.05 (Figure 4C). There were no significant differences between the EtOH only group and controls. No effects of Sex were observed.
Open field activity levels
Locomotor activity
In contrast to motor development, activity levels were most affected by the combination of EtOH and THC in a sex‐dependent manner. The total distance traveled in the open field declined across Days, F (3, 246) = 20.51, p < 0.001, and Bin, F (11, 902) = 345.70, p < 0.001, within each session. Although there were no significant main effects of EtOH, THC, or Sex, there were significant interactions of Day*EtOH*THC, F (3, 246) = 5.98, p < 0.001, Bin*THC, F (11, 902) = 1.94, p < 0.05, and a trending 5‐way interaction of Day*Bin*Sex*EtOH*THC, F (33, 2706) = 1.40, p = 0.06. Very different patterns were evident between female and male offspring; analyses were conducted separately for each Sex.
Among female offspring, separate interactions of Day*Bin*EtOH*THC, F (33, 1386) = 1.53, p < 0.05, and Bin*EtOH*THC, F (11, 462) = 2.01, p < 0.05, were observed. However, no consistent follow‐up analyses of prenatal EtOH, THC, or the combination indicated meaningful effects (Figure 5B). In contrast, among males, the combination of prenatal EtOH and THC significantly increased locomotor activity, producing significant interactions of Day*EtOH*THC, F (3, 120) = 6.02, p < 0.001, and Bin*THC, F (11, 440) = 2.31, p < 0.01. Follow‐up analyses indicated that male offspring exposed to the combination of EtOH + THC traveled significantly further on the first day of testing (PD 31; SNK p's < 0.05; Figure 5C). Within sessions, male offspring prenatally exposed to EtOH + THC also traveled farther than the other Groups toward the end of the sessions (Bins 10 and 11, p's < 0.05; Figure 5D). Similar group patterns were seen in distance traveled in the periphery of the open field, fine movement, and number of beam breaks (data not shown).
FIGURE 5.
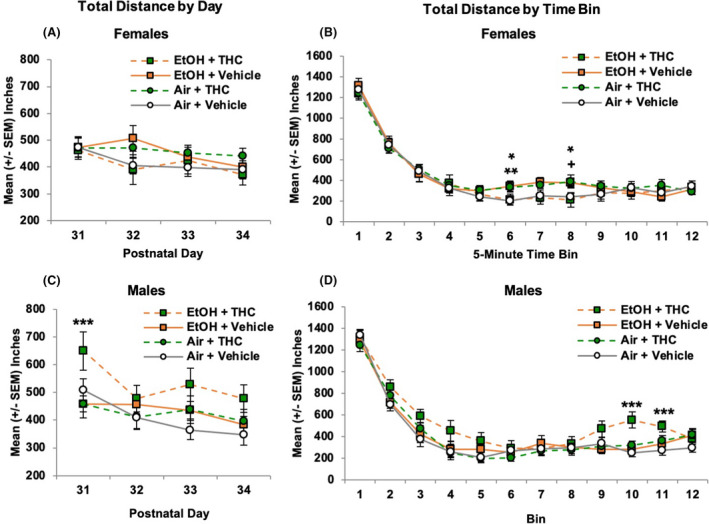
Among female offspring, prenatal EtOH or THC exposure separately increased activity levels during the middle of the sessions compared with controls (B). In contrast, combined exposure to prenatal EtOH and THC significantly increased activity levels among male offspring overall on Day 1 (C), as well as toward the end of the testing sessions (D). * = EtOH + Vehicle > Air + Vehicle, p's < 0.05. ** = Air + THC > Air + Vehicle, p < 0.05. + = Air + THC > Air + Vehicle, p = 0.06. *** = EtOH + THC > all other groups, p's < 0.05
Center distance
The effects of prenatal drug exposures were particularly striking when examining locomotor activity within the center of the chamber (inner 8 × 8 in). As expected, the center distance traveled declined across Days, F (3, 246) = 20.89, p < 0.001, and Bin within each session, F (11, 902) = 244.42, p < 0.001. There were also significant interactions of Sex*EtOH*THC, F (1, 82) = 5.28, p < 0.05, Day*EtOH*THC, F (3, 246) = 4.78, p < 0.01, and Bin*Sex, F (11, 902) = 1.83, p < 0.05.
Among female offspring, no further interactions or main effects of any variable reached significance (Figure 6A,B). In contrast, among males, there was an overall interaction of EtOH*THC, F (1, 40) = 4.18, p < 0.05, as males exposed to the combination of EtOH and THC prenatally traveled significantly further in the center of the chamber compared with all other groups. There was also an interaction of Day*EtOH*THC, F (3, 120) = 4.37, p < 0.01; the combination of EtOH and THC exposure increased locomotor activity in the chamber center on the first and third days of testing (p's < 0.05; Figure 6C), failing to reach significance on the fourth day (p = 0.07). Within sessions, this effect was largest early in the sessions (Bins 3 to 5; p's < 0.05) and toward the end of the sessions (Bins 9 to 11; p's < 0.05; Figure 6D). Similar effects were seen with the number of center entries (data not shown).
FIGURE 6.
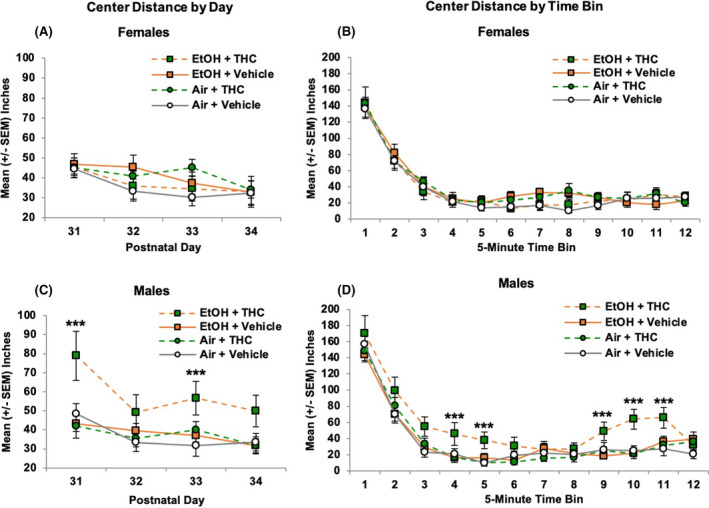
Prenatal exposure did not significantly change the distance traveled in the center of the chamber among females on any day of activity testing (A) or across testing sessions (B). However, male offspring exposed to the combination of alcohol and THC prenatally traveled greater distances on Days 1 and 3 of testing (C) and throughout the testing session durations (D), indicating impaired habituation. *** = EtOH + THC > all other groups, p < 0.05
Center time
As expected, males exposed to combined EtOH and THC also spent more time in the center of the chamber. Time spent (sec) in the center of the chamber decreased across Days, F (3, 246) = 2.88, p < 0.05, and Bins within each session, F (11, 902) = 52.64, p < 0.001. In addition, interactions of Sex*EtOH*THC, F (1, 82) = 8.40, p < 0.01, Sex*EtOH, F (1, 82) = 3.70, p = 0.05, Day*Bin*THC, F (33, 2706) = 1.56, p < 0.05, and Bin*Sex, F (11, 902) = 2.84, p < 0.01, were evident.
Among female offspring, there were no main effects of prenatal EtOH or THC exposure (Figure 7A,B). In contrast, males exposed to the combination of EtOH and THC spent more time in the center of the chamber overall, producing an interaction of EtOH*THC, F (1, 40) = 7.35, p < 0.01. There were no significant alterations with Day and Bin among males, likely due to the high amount of variability across male offspring in this group; however, increased center time in this group was most robust on Day 1 (SNK p's < 0.05; Figure 7C) and toward the end of the testing sessions (Bins 5, 9 to 11: SNK p's < 0.05; Figure 7D).
FIGURE 7.
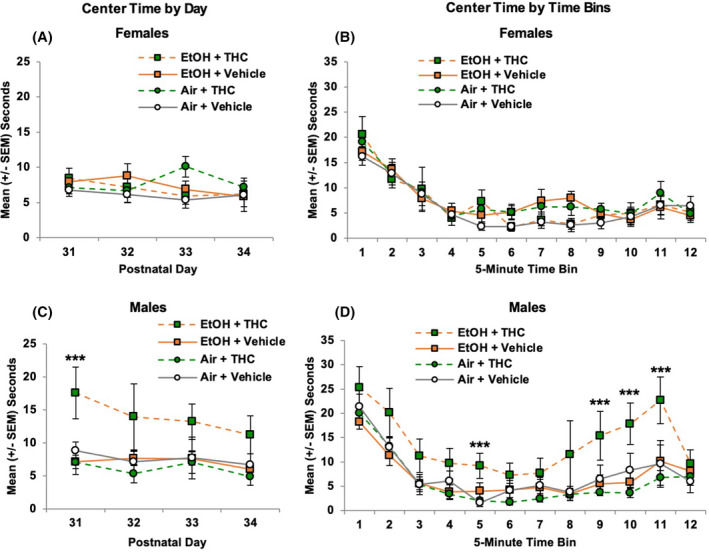
Prenatal exposure to alcohol and/or THC did not significantly alter the time spent in the center of the chamber among female offspring (A and B). However, combined exposure to alcohol and THC generally increased the center time of male offspring in the open field on Day 1 (C) and at the end of the testing sessions (D). *** = EtOH + THC > all other groups; p's < 0.05
Rearing
In contrast to locomotor activity, no main or interactive effects of Sex were observed on rearing, an exploratory behavior (Figure 8). The number of times subjects reared within the chamber declined across Days, F (3, 246) = 7.94, p < 0.001, and Bin within each session, F (11, 902) = 243.01, p < 0.001. Offspring exposed to EtOH prenatally reared less at the very beginning of the sessions (Bin 1) than Air‐exposed groups, F (1, 86) = 4.29, p < 0.05 (Figure 8B), contributing to a Bin*EtOH*THC, F (11, 902) = 2.31, p < 0.01, interaction. However, no further significant effects of prenatal exposure were observed throughout the duration of the sessions, suggesting that the alterations observed in other activity measures are not the result of a global effect on activity levels.
FIGURE 8.
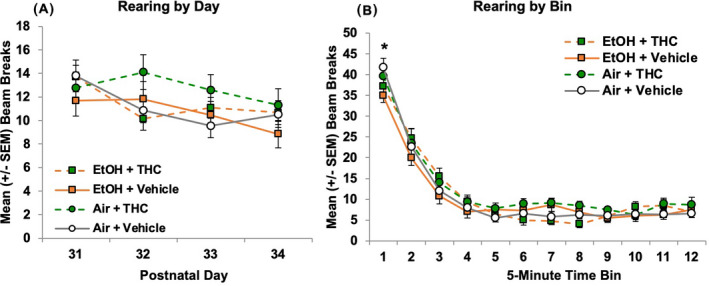
Prenatal exposure did not significantly change the frequency of rearing in the open‐field chamber among either sex (A). All offspring exposed to alcohol prenatally reared less than those exposed to air at the beginning of the session (B). * = EtOH (collapsed) < Air (collapsed), p < 0.05
DISCUSSION
This study is the first to use a novel co‐exposure model of alcohol and THC via e‐cigarettes to examine long‐term behavioral alterations in offspring. Here, we illustrate that prenatal alcohol and THC exposure via e‐cigarettes leads to both separate and interactive alterations in motor function. Prenatal THC exposure via e‐cigarettes reduced body weights during both early (PD 12 to 20) and adolescent (PD 30 to 34) periods among offspring of both sexes. In addition, prenatal THC exposure also delayed early sensorimotor development and continued to impair motor coordination during early adolescence, alone or in combination with alcohol. In contrast, prenatal alcohol exposure produced subtle and transient reductions in exploratory behaviors in the open‐field activity chambers, and both prenatal alcohol and THC alone induced modest increases in locomotor activity among females. However, only the combination of prenatal alcohol and THC exposure increased locomotor activity levels among male offspring.
We have previously established that this novel, co‐exposure model of vaporized alcohol and THC induces physiologically relevant drug levels and induces pharmacokinetic effects in the blood of pregnant rats (alcohol: 150 to 200 mg/dl; THC: 20 ng/ml) while avoiding maternal nutritional confounds and litter outcomes (Breit et al., 2020). These levels mimic a moderate binge dose of alcohol (twice the legal limit of 80 mg/dl) and a low–moderate dose of THC (Andrenyak et al., 2017) while replicating the current criteria of clinical simultaneous alcohol and cannabis use (Linden‐Carmichael et al., 2019; Patrick et al., 2018, 2019; Sokolovsky et al., 2020). Given the lasting effects on offspring behavior with these doses, the results are particularly concerning. Interestingly, combined exposure to alcohol and THC increased maternal blood levels of each drug more than either drug alone, suggesting pharmacokinetic interactions of the combination of exposures among pregnant dams, an effect similar to clinical findings following co‐consumption (Hartman et al., 2015). It is known that both alcohol and cannabis converge on endocannabinoid signaling pathways, including the CB1 receptors (Fish et al., 2019; Kunos, 2020), making it likely that interactive effects of prenatal exposure to these drugs may occur. Despite this pharmacokinetic interaction, the only behavioral interactive effects in the present study were seen in open‐field activity among males.
Although prenatal alcohol exposure delayed eye‐opening among these offspring (Breit et al., 2020), here, prenatal THC exposure consistently reduced body growth. Among the sex pair tested on the motor coordination tasks, reduced body weights were observed from PD 12 to PD 32 among both male and female offspring exposed to THC prenatally, alone or in combination with alcohol. In the sex pair tested in the open field, similar body weight reductions were seen from PD 31 to 34 in both males and females, although only reached statistical significance among offspring exposed to prenatal THC alone. Overall, prenatal THC exposure via e‐cigarettes reduced offspring body weights during the early and adolescent periods, which is similar to findings of clinical data suggesting that prenatal cannabis exposure may impair body growth (Abel, 1980).
In contrast, prenatal alcohol exposure via vapor inhalation did not alter body weight growth. Although prenatal alcohol exposure is associated with smaller birth weights and body growth in clinical data (Caputo et al., 2016), alcohol‐related reductions in offspring exposed prenatally are less consistent in preclinical data (Dursun et al., 2006; Hannigan et al., 1993; Helfer et al., 2009; Thomas et al., 2009, 2010) and are often dose‐dependent (Abel & Dintcheff, 1978). In the current study, average peak BACs among those who received alcohol alone were 150 mg/dl, whereas those in the combined group reached 200 mg/dl (Breit et al., 2020). Previous studies who have shown reduced body weights and hyperactivity among offspring used binge‐like alcohol exposure with BACs exceeding 200 to 300 mg/dl (Dursun et al., 2006; Thomas et al., 2009; Thomas et al., 2010).
However, impaired motor coordination following prenatal alcohol exposure in rodent models has been inconsistently seen even among research achieving these higher BAC levels (Driscoll et al., 1990; Dursun et al., 2006; Idrus et al., 2017; Thomas et al., 2009, 2010). While clinical data have found prenatal alcohol‐related motor impairments consistently (Driscoll et al., 1990), preclinical data have not. In addition to the timing of exposure, it is also possible that differences in timing or administrative route could also contribute to differences.
Although the effects of prenatal cannabinoid exposure on motor skill development have been similarly inconsistent in the literature (Huizink, 2014; Schneider, 2009), we observed clear delays in early sensorimotor development among offspring following prenatal THC exposure via e‐cigarettes. Prenatal THC exposure significantly delayed the age for offspring to achieve a successful trial, which is a developmental milestone in rats. Given that this difference was specifically observed in the first day of success rather than the total number of successes, this suggests that a potential learning effect was not a confound in these results. Although offspring with prenatal THC exposure showed this delay in the first couple of days of testing (PD 12 to 13), it is important to note that they did eventually catch up to the performance of other groups in this task (PD 15). However, these same offspring showed impairments on a motor task that requires coordination and balance later in life, from PD 30 to 32. Offspring exposed to prenatal THC required significantly more trials to successfully traverse the parallel bars and had a lower success ratio on the first day of the task, achieving lower maximum widths between bars. These impairments in early motor development and adolescent motor coordination following prenatal THC exposure were not exacerbated by alcohol, nor were they dependent on sex, suggesting an overall motor impairment induced by prenatal exposure to THC via e‐cigarettes. It will be important for future studies to examine the mechanism(s) by which prenatal THC exposure alters motor development and coordination.
These results differ greatly from our previous study illustrating that combined alcohol and a synthetic cannabinoid (CP‐55,940) during the brain growth spurt advances motor development, with long‐term motor impairments in females (Breit et al., 2019a). In addition to the differing cannabinoids, levels, and routes, these studies also varied in developmental timing of exposure, which would impact different aspects of cannabinoid receptor development in several motor‐related brain areas. For example, cannabinoid type 1 (CB1) receptors play roles in embryonal implantation, neuronal development, and early synaptic communication as early as GD 11 to 14 in rodents (Harkany et al., 2007); during this time, prenatal cannabis exposure may impair early developmental processes, including motor function. In the present study, exposure occurred between GD 5 and 20, consistent with clinical and preclinical studies reporting that prenatal cannabinoid exposure is associated with decreased early motility (Fried, 1976), impaired early motor skills (Richardson et al., 1995), and impaired motor coordination (Shabani et al., 2011). In contrast, cannabinoid exposure later in development may up‐regulate GABA activity and enhance performance (Benagiano et al., 2007), consistent with the advanced sensorimotor development we found with administration with a synthetic cannabinoid during the human third‐trimester equivalent (Breit et al., 2019a) and other clinical studies (Fried & Watkinson, 1990). Importantly, fetal development requires a careful timing of events, and any changes in neuronal development can lead to later‐life consequences (Wolosker et al., 2008). Thus, the overall conclusion is that prenatal cannabis exposure may alter motor development and coordination among offspring, and those alterations may vary based on the timing of administration.
In contrast, distinct interactive effects of prenatal alcohol and THC exposure via e‐cigarettes were seen in the open‐field activity chambers. Both clinical and preclinical data examining the effects of cannabinoid exposure during early development on activity levels are mixed, with data in each field supporting either increased activity (Borgen et al., 1973; Breit et al., 2019b; Fried et al., 1992; Goldschmidt et al., 2000; Mereu et al., 2003; Navarro et al., 1994; Rubio et al., 1995) or no change (Brake et al., 1987; Fried & Watkinson, 1988; Hutchings et al., 1989; Vardaris et al., 1976). Prenatal alcohol exposure has been linked to greater levels of attention deficit hyperactivity disorder symptoms (Popova et al., 2016) and increased hyperactivity in rodents (Bond, 1981; Breit et al., 2019b; Ryan et al., 2008; Thomas et al., 2007), similar to clinical findings among individuals with FASD (Mattson et al., 2011). The current study found modest increases in locomotor activity following either prenatal alcohol or THC among females. However, only the combination of prenatal alcohol and THC increased activity levels among males. This result is similar to our previous work showing that early developmental exposure to either alcohol or a synthetic cannabinoid (CP‐55,940) during the brain growth spurt increased adolescent activity levels, but that the combination exacerbated hyperactivity among offspring by reducing habituation in locomotor activity during the testing session (Breit et al., 2019b). Importantly, in both studies, co‐exposure to alcohol and cannabinoids increased BACs more than the alcohol dose alone in both pregnant dams (Breit et al., 2020) and neonates (Breit et al., 2019b). Given that the severity of FASD may be dose‐dependent (Maier & West, 2001), it is possible that the interactive effects of alcohol and cannabinoid exposure on activity levels could be related to higher BACs. Research examining the effects of combined prenatal alcohol and cannabis exposure on long‐term behavioral development among offspring is incredibly sparse, and this possibility should be further examined using dose‐dependent studies.
Not only did combined prenatal exposure to alcohol and THC increase overall activity levels, but interactive effects were also seen in behaviors specifically in the center of the open‐field chambers. Males prenatally exposed to the combination of alcohol and THC did not habituate to the center of the chambers at the same rate, and the increases in activity observed toward the end of the session may reflect recovery from habituation. In addition, combined exposure to alcohol and THC during gestation increased the number of entries into the center, time spent in the center, and the distance traveled in the center of the chambers among male offspring. Rodents are naturally aversive to open spaces, and thus increased center‐related activities could be a by‐product of overall increased locomotor activity, but could also be related to alterations in anxiety‐related behaviors such as decreased risk assessment or increased impulsivity (Prut & Belzung, 2003). Importantly, the effects observed in the center arena measures in the open‐field activity chambers do not mirror the behavioral alterations observed in the other motor‐related tasks, suggesting that the impairment in motor development and coordination seen among offspring exposed to THC prenatally are not driven by changes in anxiety‐related behaviors. Moreover, given that the same effect was not observed in rearing, this suggests that the observed activity‐related alterations on distance traveled and center behaviors are not a global effect on activity levels.
In the current study, it is unclear why the interactive effect of prenatal alcohol and THC exposure on certain activity measures was only observed among males, given that there is very little known about the effects of combined prenatal alcohol and cannabis exposure in general. However, some clinical (Ouellet‐Morin et al., 2011) and preclinical data (Hellemans et al., 2010) do suggest that males are more susceptible to these types of alterations in activity levels and anxiety‐related domains following prenatal alcohol exposure compared with females. The origins of this sex difference will be important to further examine in future studies.
There are several limitations to acknowledge in this study. One limitation was that it utilized single doses of both alcohol and THC; it will be important to understand how wider dose ranges influence behavioral development. It will also be important to identify which doses provide initial synergistic interactions. Although these doses are not as high as those published in substance abuse studies, effects of moderate doses are important to research since they represent what is more commonly used among women of child‐bearing age. In addition, this study focused on THC, the primary psychoactive component of cannabis, but there are more than 500 chemical compounds of cannabinoids (Radwan et al., 2017). Thus, we acknowledge the outcomes of this study may not generalize to all types of prenatal cannabis exposure. In addition, there are a variety of behavioral tasks that measure motor‐ and activity‐related behaviors, as well as apparatus variations among those used in this study; thus, the differences we observed can only be attributed to the behavioral methodology described here. Also, the control group in this study was exposed to the vehicle, propylene glycol, which contains toxic compounds and could potentially impact neurodevelopment (Strongin, 2019). As mentioned in our previous publication, we did include a small pilot group of nonhandled, non‐vape‐exposed controls and found no differences in any maternal factors (Breit et al., 2020) or the offspring behaviors described in this study compared to the Air + Vehicle controls. However, it will be important for future studies to consider the possibility that propylene glycol may exert independent effects. Lastly, although e‐cigarettes are a popular route of administration for THC, including among pregnant women, we recognize that vapor inhalation is not as clinically relevant of an administration route for alcohol. We chose to expose pregnant dams to alcohol via vapor inhalation since they would already be exposed to vapor via e‐cigarettes, and we wanted to minimize additional stress. Importantly, we found similar effects using this alcohol vapor inhalation paradigm (Breit et al., 2020) on maternal weight gain and offspring eye‐opening as we have with maternal intragastric intubation (Thomas et al., 2009). In addition, we have also found similar pharmacokinetic effects with combined alcohol and synthetic cannabinoid exposure via neonatal alcohol intubations and cannabinoid intraperitoneal injections (Breit et al., 2019a).
In summary, these results suggest that prenatal exposure to alcohol and THC via e‐cigarettes has domain‐specific effects on motor‐related behaviors. Prenatal THC exposure decreased offspring body weights throughout early and adolescent development, regardless of sex. In addition, prenatal THC exposure delayed both early sensorimotor development and adolescent motor coordination. Prenatal alcohol exposure at this dose did not alter body growth, motor development, or motor coordination and produced modest effects on activity level. Combined prenatal exposure to these drugs did not have additive effects on sensorimotor development. In contrast, combined prenatal exposure to alcohol and THC increased activity levels. Taken together, prenatal cannabis exposure may lead to impaired motor performance on its own, whereas combined exposure with alcohol may additionally lead to hyperactivity and altered impulsivity. Since alcohol and cannabis are often consumed together and nearly 40% of pregnancies in the United States are unplanned (Mosher et al., 2012), these results have important implications for informing individuals who may become pregnant regarding the potential consequences of co‐use.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
This work was supported by NIAAA grants AA025425 and AA012446 to Dr. Jennifer D. Thomas, a NIAAA training grant T32 AA007456‐38 to Dr. Kristen R. Breit, and a NIH Loan Repayment Program award to Dr. Kristen R. Breit. Special thanks to Maury Cole at La Jolla Alcohol Research, Inc. (San Diego, CA) for building the vapor inhalation equipment and Dr. Michael Taffe for vapor inhalation advisement. Thank you to the NIDA Drug Supply for providing all THC used in this study. Lastly, we want to recognize members of the Center for Behavioral Teratology at San Diego State University for assisting in data collection and interpretation, particularly the instrumental efforts of Brandonn Zamudio, Ivette Gonzalez, Bahar Sabouri, and Valery Quinonez.
Breit, K.R. , Rodriguez, C. G. , Lei, A. , Hussain, S. & Thomas, J.D. (2022) Effects of prenatal alcohol and delta‐9‐tetrahydrocannabinol exposure via electronic cigarettes on motor development. Alcoholism: Clinical and Experimental Research, 46, 1408–1422. Available from: 10.1111/acer.14892
REFERENCES
- Abel, E. & Subramanian, M. (1990) Effects of low doses of alcohol on delta‐9‐tetrahydrocannabinol's effects in pregnant rats. Life Sciences, 47, 1677–1682. [DOI] [PubMed] [Google Scholar]
- Abel, E. , Tan, S. & Subramanian, M. (1987) Effects of Δ9‐tetrahydrocannabinol, phenobarbital, and their combination on pregnancy and offspring in rats. Teratology, 36, 193–198. [DOI] [PubMed] [Google Scholar]
- Abel, E.L. (1980) Prenatal exposure to cannabis: a critical review of effects on growth, development, and behavior. Behavioral and Neural Biology, 29, 137–156. [DOI] [PubMed] [Google Scholar]
- Abel, E.L. & Dintcheff, B.A. (1978) Effects of prenatal alcohol exposure on growth and development in rats. Journal of Pharmacology and Experimental Therapeutics, 207, 916–921. [PubMed] [Google Scholar]
- Agnish, N.D. & Keller, K.A. (1997) The rationale for culling of rodent litters. Fundamental and Applied Toxicology, 38, 2–6. [DOI] [PubMed] [Google Scholar]
- Andrenyak, D.M. , Moody, D.E. , Slawson, M.H. , O'leary, D.S. & Haney, M. (2017) Determination of delta‐9‐tetrahydrocannabinol (THC), 11‐hydroxy‐THC, 11‐nor‐9‐carboxy‐THC and cannabidiol in human plasma using gas chromatography–tandem mass spectrometry. Journal of Analytical Toxicology, 41, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa, B.S. , Ninan, I. & Arancio, O. (2008) Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. Journal of Neurochemistry, 107, 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano, V. , Lorusso, L. , Flace, P. , Girolamo, F. , Rizzi, A. , Sabatini, R. et al. (2007) Effects of prenatal exposure to the CB‐1 receptor agonist WIN 55212‐2 or CO on the GABAergic neuronal systems of rat cerebellar cortex. Neuroscience, 149, 592–601. [DOI] [PubMed] [Google Scholar]
- Bond, N.W. (1981) Prenatal alcohol exposure in rodents: A review of its effects on offspring activity and learning ability. Australian Journal of Psychology, 33, 331–344. [Google Scholar]
- Borgen, L.A. , Davis, W.M. & Pace, H.B. (1973) Effects of prenatal delta‐9‐tetrahydrocannabinol on the development of rat offspring. Pharmacology Biochemistry and Behavior, 1, 203–206. [Google Scholar]
- Brake, S.C. , Hutchings, D.E. , Morgan, B. , Lasalle, E. & Shi, T. (1987) Delta‐9‐tetrahydrocannabinol during pregnancy in the rat: II. Effects on ontogeny of locomotor activity and nipple attachment in the offspring. Neurotoxicology and Teratology, 9, 45–49. [DOI] [PubMed] [Google Scholar]
- Breit, K.R. , Rodriguez, C. , Lei, A. & Thomas, J.D. (2020) Combined vapor exposure to THC and alcohol in pregnant rats: maternal outcomes and pharmacokinetic effects. Neurotoxicology and Teratology, 82, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit, K.R. , Zamudio, B. & Thomas, J.D. (2019a) Altered motor development following late gestational alcohol and cannabinoid exposure in rats. Neurotoxicology and Teratology, 73, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit, K.R. , Zamudio, B. & Thomas, J.D. (2019b) The effects of alcohol and cannabinoid exposure during the brain growth spurt on behavioral development in rats. Birth Defects Research, 111, 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo, C. , Wood, E. & Jabbour, L. (2016) Impact of fetal alcohol exposure on body systems: a systematic review. Birth Defects Research Part C: Embryo Today: Reviews, 108, 174–180. [DOI] [PubMed] [Google Scholar]
- Cardenas, V.M. , Fischbach, L.A. & Chowdhury, P. (2019) The use of electronic nicotine delivery systems during pregnancy and the reproductive outcomes: A systematic review of the literature. Tobacco Induced Diseases, 17, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahoud, I. & Paumgartten, F.J. (2009) Influence of litter size on the postnatal growth of rat pups: is there a rationale for litter‐size standardization in toxicity studies? Environmental Research, 109, 1021–1027. [DOI] [PubMed] [Google Scholar]
- Downey, L.A. , King, R. , Papafotiou, K. , Swann, P. , Ogden, E. , Boorman, M. et al. (2013) The effects of cannabis and alcohol on simulated driving: influences of dose and experience. Accident Analysis & Prevention, 50, 879–886. [DOI] [PubMed] [Google Scholar]
- Driscoll, C.D. , Streissguth, A.P. & Riley, E.P. (1990) Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicology and Teratology, 12, 231–237. [DOI] [PubMed] [Google Scholar]
- Dursun, I. , Jakubowska‐Doğru, E. & Uzbay, T. (2006) Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacology Biochemistry and Behavior, 85, 345–355. [DOI] [PubMed] [Google Scholar]
- Festing, M.F. (2006) Design and statistical methods in studies using animal models of development. ILAR Journal, 47, 5–14. [DOI] [PubMed] [Google Scholar]
- Fish, E.W. , Murdaugh, L.B. , Zhang, C. , Boschen, K.E. , Boa‐Amponsem, O. , Mendoza‐Romero, H.N. et al. (2019) Cannabinoids exacerbate alcohol teratogenesis by a CB1‐hedgehog interaction. Scientific Reports, 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried, P. (1976) Short and long‐term effects of pre‐natal cannabis inhalation upon rat offspring. Psychopharmacology, 50, 285–291. [DOI] [PubMed] [Google Scholar]
- Fried, P. & Watkinson, B. (1988) 12‐and 24‐month neurobehavioural follow‐up of children prenatally exposed to marihuana, cigarettes and alcohol. Neurotoxicology and Teratology, 10, 305–313. [DOI] [PubMed] [Google Scholar]
- Fried, P.A. & Watkinson, B. (1990) 36‐and 48‐month neurobehavioral follow‐up of children prenatally exposed to marijuana, cigarettes, and alcohol. Journal of Developmental and Behavioral Pediatrics, 11, 49–58. [PubMed] [Google Scholar]
- Fried, P.A. , Watkinson, B. & Gray, R. (1992) A follow‐up study of attentional behavior in 6‐year‐old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicology and Teratology, 14, 299–311. [DOI] [PubMed] [Google Scholar]
- Goldschmidt, L. , Day, N.L. & Richardson, G.A. (2000) Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicology and Teratology, 22, 325–336. [DOI] [PubMed] [Google Scholar]
- Goldschmidt, L. , Richardson, G.A. , Cornelius, M.D. & Day, N.L. (2004) Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicology and Teratology, 26, 521–532. [DOI] [PubMed] [Google Scholar]
- Hannigan, J.H. , Berman, R.F. & Zajac, C.S. (1993) Environmental enrichment and the behavioral effects of prenatal exposure to alcohol in rats. Neurotoxicology and Teratology, 15, 261–266. [DOI] [PubMed] [Google Scholar]
- Hansen, H.H. , Krutz, B. , Sifringer, M. , Stefovska, V. , Bittigau, P. , Pragst, F. et al. (2008) Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 64, 42–52. [DOI] [PubMed] [Google Scholar]
- Harkany, T. , Guzman, M. , Galve‐Roperh, I. , Berghuis, P. , Devi, L.A. & Mackie, K. (2007) The emerging functions of endocannabinoid signaling during CNS development. Trends in Pharmacological Sciences, 28, 83–92. [DOI] [PubMed] [Google Scholar]
- Hartman, R.L. , Brown, T.L. , Milavetz, G. , Spurgin, A. , Gorelick, D.A. , Gaffney, G. et al. (2015) Controlled cannabis vaporizer administration: blood and plasma cannabinoids with and without alcohol. Clinical Chemistry, 61, 850–869. [DOI] [PubMed] [Google Scholar]
- Hedden, S.L. (2015). Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, Department of Heath & Human Services.
- Helfer, J.L. , Goodlett, C.R. , Greenough, W.T. & Klintsova, A.Y. (2009) The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Research, 1294, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans, K.G. , Verma, P. , Yoon, E. , Yu, W.K. , Young, A.H. & Weinberg, J. (2010) Prenatal alcohol exposure and chronic mild stress differentially alter depressive‐and anxiety‐like behaviors in male and female offspring. Alcoholism: Clinical and Experimental Research, 34, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink, A. (2014) Prenatal cannabis exposure and infant outcomes: overview of studies. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 52, 45–52. [DOI] [PubMed] [Google Scholar]
- Hutchings, D.E. , Gamagaris, Z. , Miller, N. & Fico, T.A. (1989) The effects of prenatal exposure to delta‐9‐tetrahydrocannabinol on the rest‐activity cycle of the preweanling rat. Neurotoxicology and Teratology, 11, 353–356. [DOI] [PubMed] [Google Scholar]
- Idrus, N.M. , Breit, K.R. & Thomas, J.D. (2017) Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicology and Teratology, 59, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlenski, M. , Koma, J.W. , Zank, J. , Bodnar, L.M. , Bogen, D.L. & Chang, J.C. (2017) Trends in perception of risk of regular marijuana use among US pregnant and nonpregnant reproductive‐aged women. American Journal of Obstetrics and Gynecology, 217, 705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.G. , Moon, J. , Dixon, H. & Tullar, P. (2018) Recurrent nausea and vomiting in a pregnant woman with chronic marijuana use. Case Reports in Obstetrics and Gynecology, 2018, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos, G. (2020) Interactions between alcohol and the endocannabinoid system. Alcoholism: Clinical and Experimental Research, 44, 790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, S. , Shield, K. , Koren, G. , Rehm, J. & Popova, S. (2014) A comparison of the prevalence of prenatal alcohol exposure obtained via maternal self‐reports versus meconium testing: A systematic literature review and meta‐analysis. BMC Pregnancy and Childbirth, 14, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic, S.E. & Essioux, L. (2013) Improving basic and translational science by accounting for litter‐to‐litter variation in animal models. BMC Neuroscience, 14, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden‐Carmichael, A.N. , Stamates, A.L. & Lau‐Barraco, C. (2019) Simultaneous use of alcohol and marijuana: patterns and individual differences. Substance Use & Misuse, 54, 2156–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, S.E. & West, J.R. (2001) Drinking patterns and alcohol‐related birth defects. Alcohol Research & Health, 25, 168–174. [PMC free article] [PubMed] [Google Scholar]
- Mark, K.S. , Farquhar, B. , Chisolm, M.S. , Coleman‐Cowger, V.H. & Terplan, M. (2015) Knowledge, attitudes, and practice of electronic cigarette use among pregnant women. Journal of Addiction Medicine, 9, 266–272. [DOI] [PubMed] [Google Scholar]
- Mattson, S.N. , Crocker, N. & Nguyen, T.T. (2011) Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology Review, 21, 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu, G. , Fà, M. , Ferraro, L. , Cagiano, R. , Antonelli, T. , Tattoli, M. et al. (2003) Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long‐term potentiation and glutamate release. Proceedings of the National Academy of Sciences, 100, 4915–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher, W. D. , Jones, J. & Abma, J. C. 2012. Intended and uninteded Births in the United States: 1982‐2010. National Health Statistics Report. www.cdc.gov: Centers for DIsease Control and Prevention. [PubMed]
- Nagre, N.N. , Subbanna, S. , Shivakumar, M. , Psychoyos, D. & Basavarajappa, B.S. (2015) CB 1‐receptor knockout neonatal mice are protected against ethanol‐induced impairments of DNMT 1, DNMT 3A, and DNA methylation. Journal of Neurochemistry, 132, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, M. , De Fonseca, F.R. , Hernandez, M. , Ramos, J. & Fernandez‐Ruiz, J. (1994) Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmacology Biochemistry and Behavior, 47, 47–58. [DOI] [PubMed] [Google Scholar]
- O'malley, K.D. & Nanson, J. (2002) Clinical implications of a link between fetal alcohol spectrum disorder and attention‐deficit hyperactivity disorder. The Canadian Journal of Psychiatry, 47, 349–354. [DOI] [PubMed] [Google Scholar]
- Ouellet‐Morin, I. , Dionne, G. , Lupien, S.J. , Muckle, G. , Côté, S. , Pérusse, D. et al. (2011) Prenatal alcohol exposure and cortisol activity in 19‐month‐old toddlers: an investigation of the moderating effects of sex and testosterone. Psychopharmacology, 214, 297–307. [DOI] [PubMed] [Google Scholar]
- Patrick, M.E. , Fairlie, A.M. , Cadigan, J.M. , Abdallah, D.A. , Larimer, M.E. & Lee, C.M. (2019) Daily motives for alcohol and marijuana use as predictors of simultaneous use among young adults. Journal of Studies on Alcohol and Drugs, 80, 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick, M.E. , Fairlie, A.M. & Lee, C.M. (2018) Motives for simultaneous alcohol and marijuana use among young adults. Addictive Behaviors, 76, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova, S. , Lange, S. , Shield, K. , Mihic, A. , Chudley, A.E. , Mukherjee, R.A. et al. (2016) Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta‐analysis. The Lancet, 387, 978–987. [DOI] [PubMed] [Google Scholar]
- Prut, L. & Belzung, C. (2003) The open field as a paradigm to measure the effects of drugs on anxiety‐like behaviors: a review. European Journal of Pharmacology, 463, 3–33. [DOI] [PubMed] [Google Scholar]
- Radwan, M.M. , Wanas, A.S. , Chandra, S. & Elsohly, M.A. (2017) Natural cannabinoids of cannabis and methods of analysis. In: Cannabis Sativa L.‐ Botany and Biotechnology. Cham: Springer, pp. 161–182. [Google Scholar]
- Richardson, G.A. , Day, N.L. & Goldschmidt, L. (1995) Prenatal alcohol, marijuana, and tobacco use: infant mental and motor development. Neurotoxicology and Teratology, 17, 479–487. [DOI] [PubMed] [Google Scholar]
- Rubio, P. , De Fonseca, F.R. , Muñoz, R. , Ariznavarreta, C. , Martín‐Calderón, J. & Navarro, M. (1995) Long‐term behavioral effects of perinatal exposure to delta‐9‐tetrahydrocannabinol in rats: possible role of pituitaryadrenal axis. Life Sciences, 56, 2169–2176. [DOI] [PubMed] [Google Scholar]
- Ryan, S.H. , Williams, J.K. & Thomas, J.D. (2008) Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Research, 1237, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, M. (2009) Cannabis use in pregnancy and early life and its consequences: animal models. European Archives of Psychiatry and Clinical Neuroscience, 259, 383–393. [DOI] [PubMed] [Google Scholar]
- Shabani, M. , Hosseinmardi, N. , Haghani, M. , Shaibani, V. & Janahmadi, M. (2011) Maternal exposure to the CB1 cannabinoid agonist WIN 55212‐2 produces robust changes in motor function and intrinsic electrophysiological properties of cerebellar purkinje neurons in rat offspring. Neuroscience, 172, 139–152. [DOI] [PubMed] [Google Scholar]
- Sokolovsky, A.W. , Gunn, R.L. , Micalizzi, L. , White, H.R. & Jackson, K.M. (2020) Alcohol and marijuana co‐use: consequences, subjective intoxication, and the operationalization of simultaneous use. Drug and Alcohol Dependence, 212, 107986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin, R.M. (2019) E‐cigarette chemistry and analytical detection. Annual Review of Analytical Chemistry, 12, 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbanna, S. , Shivakumar, M. , Psychoyos, D. , Xie, S. & Basavarajappa, B.S. (2013) Anandamide–CB1 receptor signaling contributes to postnatal ethanol‐induced neonatal neurodegeneration, adult synaptic, and memory deficits. Journal of Neuroscience, 33, 6350–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter, M.A. , Mastrobattista, J. , Sachs, M. & Aagaard, K. (2015) Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Research Part A: Clinical and Molecular Teratology, 103, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki, L.S. , Gomez, J. & Schwarz, J.M. (2016) An examination of sex differences in the effects of early‐life opiate and alcohol exposure. Philosophical Transactions of the Royal Society B: Biological Sciences, 371, 20150123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J.D. , Abou, E.J. & Dominguez, H.D. (2009) Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicology and Teratology, 31, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J.D. , Biane, J.S. , O'bryan, K.A. , O'neill, T.M. & Dominguez, H.D. (2007) Choline supplementation following third‐trimester‐equivalent alcohol exposure attenuates behavioral alterations in rats. Behavioral Neuroscience, 121, 120–130. [DOI] [PubMed] [Google Scholar]
- Thomas, J.D. , Garrison, M. & O'neill, T.M. (2004) Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicology and Teratology, 26, 35–45. [DOI] [PubMed] [Google Scholar]
- Thomas, J.D. , Idrus, N.M. , Monk, B.R. & Dominguez, H.D. (2010) Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Research Part A: Clinical and Molecular Teratology, 88, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardaris, R.M. , Weisz, D.J. , Fazel, A. & Rawitch, A.B. (1976) Chronic administration of delta‐9‐tetrahydrocannabinol to pregnant rats: studies of pup behavior and placental transfer. Pharmacology Biochemistry and Behavior, 4, 249–254. [DOI] [PubMed] [Google Scholar]
- Volkow, N.D. , Han, B. , Compton, W.M. & Mccance‐Katz, E.F. (2019) Self‐reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA, 322, 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright, P.E. (1998) Issues of design and analysis relating to the use of multiparous species in developmental nutritional studies. The Journal of Nutrition, 128, 661–663. [DOI] [PubMed] [Google Scholar]
- Weinberg, J. , Sliwowska, J.H. , Lan, N. & Hellemans, K. (2008) Prenatal alcohol exposure: Foetal programming, the hypothalamic‐pituitary‐adrenal axis and sex differences in outcome. Journal of Neuroendocrinology, 20, 470–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker, H. , Dumin, E. , Balan, L. & Foltyn, V.N. (2008) D‐amino acids in the brain: D‐serine in neurotransmission and neurodegeneration. The FEBS Journal, 275, 3514–3526. [DOI] [PubMed] [Google Scholar]
- Young‐Wolff, K.C. , Adams, S.R. , Wi, S. , Weisner, C. & Conway, A. (2020) Routes of cannabis administration among females in the year before and during pregnancy: results from a pilot project. Addictive Behaviors, 100, 106125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young‐Wolff, K.C. , Tucker, L.‐Y. , Alexeeff, S. , Armstrong, M.A. , Conway, A. , Weisner, C. et al. (2017) Trends in self‐reported and biochemically tested marijuana use among pregnant females in California from 2009‐2016. JAMA, 318, 2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]


