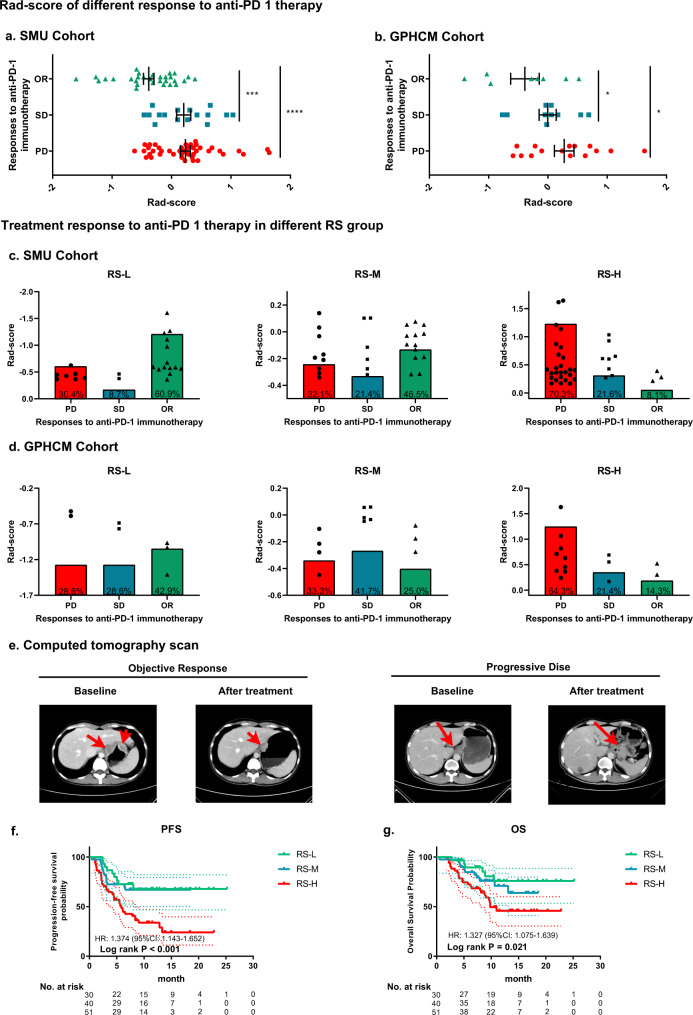
Fig. 5. Performance of the radiomics imaging biomarker in evaluating the response to anti-PD-1 immunotherapy and clinical outcomes of immunotherapy.
a Rad-score of different responses to anti-PD-1 immunotherapy in the SMU cohort (OR: n = 30; SD: n = 16; PD: n = 42). b Rad-score of different responses to anti-PD-1 immunotherapy in the GPHCM cohort (OR: n = 8; SD: n = 10; PD: n = 15); The data are presented as the mean values with SEM. For statistical comparisons among different groups, a two-tailed t test (unpaired) was used. c Rad-scores of patients and proportions of anti-PD-1 immunotherapy responses in different RS groups of the SMU cohort. d Rad-scores of patients and proportions of anti-PD-1 immunotherapy response in different RS groups of the GPHCM cohort. e CT images and changes in tumor volume of different responses to anti-PD-1 immunotherapy. f Progression-free survival of different RS groups in GC patients treated with anti-PD-1 immunotherapy. g Overall survival of different RS groups in GC patients treated with anti-PD-1 immunotherapy. Comparisons of the above progression survival curves were performed with a two-sided log-rank test. Dashed lines around the survival curves represent 95% confidence intervals. OR: objective response, SD: stable disease, PD: progressive disease, RS-L: RS-Low group, RS-M: RS-Middle group, RS-H: RS-High group, HR: hazard ratio, *P < 0.05, ***P < 0.001, ****P < 0.0001. Source data are provided as a Source data file.