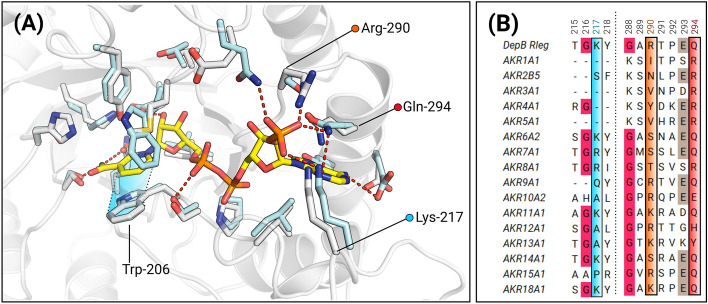
Figure 4.
Model of the coenzyme binding site highlighting the conservation of putative coenzyme binding residues. (A) NADP+ was modeled by superimposing the ternary complex of AKR6A2 with apo-DepBRleg. White sticks represent the residues from DepBRleg complex, while cyan sticks are residues corresponding to AKR6A2. Trp-206 has been re-oriented in the apo-DepBRleg structure to prevent a steric clash with the nicotinamide ring of NADP+. π-stacking interactions between the nicotinamide ring and Trp-206 are shaded in blue. In apo-DepBRleg, Lys-217 points away from the entrance of the coenzyme binding pocket. However, by analogy to Lys-254 in AKR6A2, this residue may interact with at least one of the oxygen atoms of 2’monophosphate. (B) MSA depicting conservation of residues near the 2’monophosphate of the modeled NADP+ (See Supplementary Table S1 for accession codes).