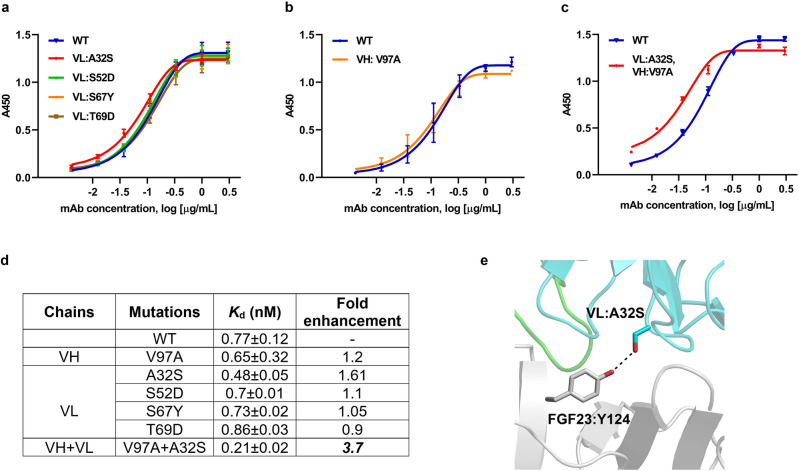
Figure 5.
Binding activity of Burosumab variants designed to enhance affinity based on pose27. Four mutations on the light chain were created and tested for binding to wild-type FGF23 using ELISA (a). Only A32S mutations showed 1.6-fold affinity enhancement based on Kd value compared with wild-type Burosumab. V97A, which showed small affinity enhancement (1.2-fold) from alanine scanning promoted the binding affinity up to 3.7-fold when combined with A32S (b,c,d). VL:A32S is predicted to form a new favorable hydrogen bond formation with Y124 of FGF23 (e). Curves were drawn by GraphPad Prism version 8.4.3. A450 refers to absorbance at 450 nm. Structure was created by PyMOL version 2.2.0.