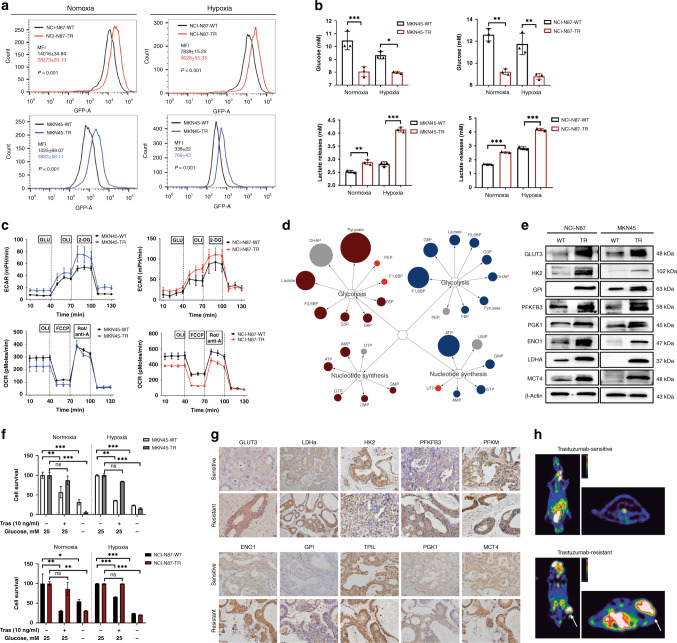
Fig. 1. Increased glucose metabolism fuels trastuzumab resistance in trastuzumab-resistant HER2 + gastric cancer.
a, b Relative glucose consumption (a), glucose residual and lactate release (b) in cultural supernatant in TRs and WTs after culturing under normoxia (20% oxygen) or hypoxia (1% oxygen) for 24 h. c The extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) in freshly isolated TRs and WTs using the Seahorse extracellular flux analyser. d Major differential metabolite pools in the glycolytic and nucleotide synthesis pathway in TRs compared with WTs. Coloured circles represent statistically significant changes. Dark red and blue circles represent upregulations; grey circles represent downregulations (P ≤ 0.05 and fold change >1). Bright-red circles represent regulations with P > 0.05. The diameter of the circles represents the degree of change compared TRs with WTs. e Western blot for glycolytic genes. f Effect of glucose deprivation on cells proliferation. The TRs and corresponding WTs were cultured in normal and low glucose conditions (0.5 mM glucose) for a short period (48 h), followed by MTT assays. g Immunohistochemistry (IHC) of genes involved in glucose metabolism in lesions of trastuzumab-resistant PDX groups compared with trastuzumab-sensitive groups. Scale bar, 100 μm (×40). h Representative images of 18F-FDG uptake imaging in trastuzumab-sensitive and resistant patient-derived tumour xenograft (PDX) models (n = 6/group). Normalised uptake values fold change for xenografts examined by FDG-PET are presented in the graph on the right. Each experiment was conducted three times and the results are presented as mean ± SEM. Student’s t test, one-way ANOVA or two-way ANOVA was used to analyse the data (*P < 0.05, **P < 0.01, ***P < 0.001).