Table 2.
Overall results obtained for 108 blood samples that confirmed the HTLV-1/2 infection in patients infected with HIV-1.
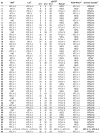 |
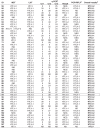 |
Samples with discordant results on confirmatory assays are depicted in a box.
ID, identification number of the sample; Ct, cycle threshold value; Ct1, cycle threshold (pol) HTLV-1; Ct2, cycle threshold (pol) HTLV-2; Ct3, cycle threshold of albumin (control); NEG, negative; IND, indeterminate; ND, not done; HTLV, human T lymphotropic virus; HTLV-1, human T lymphotropic virus type 1; HTLV-2, human T lymphotropic virus type 2.
a Results according to criteria described in Materials and Methods.
b Overall results obtained by WB and/or LIA and/or q-PCR and/or PCR-RFLP assays [HTLV Blot 2.4 (WB), MP Biomedicals, Solon, OH, USA; INNO-LIA HTLV I/II (LIA), Fujirebio, Europe N.V., Belgium; in-house molecular assays, real-time PCR pol (qPCR) and restriction fragment length polymorphism analysis of amplification products of tax nested-PCR (PCR-RFLP)].
