Abstract
Introduction and aim
Hepatitis C is a key challenge to public health in Brazil. The objective of this paper was to describe the Brazilian strategy for hepatitis C to meet the 2030 elimination goal proposed by World Health Organization (WHO).
Methods
A mathematical modeling approach was used to estimate the current HCV-infected Brazilian population, and to evaluate the relative costs of two different scenarios to address HCV disease burden in Brazil: (1) if no further changes are made to the HCV treatment program in Brazil; (2) where the WHO targets for 2030 elimination are met through diagnosis and treatment efforts peaking before 2024.
Results
An anti-HCV prevalence of 0.53% was calculated for the total population. It was estimated that the number of HCV-RNA+ individuals in Brazil in 2017 was 632,000 (0.31% of the population). Scale-up of treatment and diagnosis over time will be necessary in order to achieve WHO targets beginning in 2018. Direct costs (diagnostic, treatment and healthcare costs) are projected to increase significantly during the scale-up of treatment and diagnosis in the initial years of the intervention scenario, but then fall below the base case on an annual basis by 2025–2036, once HCV is eliminated, due to health sectors savings from the prevention of HCV liver-related morbidity and mortality.
Conclusion
Achieving the WHO targets is technically feasible in Brazil with a scale-up of treatment and diagnosis over time, beginning in 2018. However, elimination of hepatitis C requires policy changes to substantially scale-up prevention, screening and treatment of HCV, together with public health advocacy to raise awareness among affected populations and healthcare providers.
Keywords: Hepatitis C, Hepatitis C elimination, Hepatitis C disease burden, Brazil
Hepatitis C is the major cause of death among viral hepatitis infected patients in Brazil and represents one of the key challenges to public health in the country.1 The introduction of interferon-free direct-acting antivirals (DAAs), with its high rates of sustained virological response, has made feasible the elimination of hepatitis C as a global epidemic, as recommended by the World Health Organization (WHO).2
In Brazil, the public health system provides free-of-charge and universal treatment for all HCV-infected patients, according to the principles of the country's Unified Health System (SUS), which was instituted by the Federal Constitution of 1988 based on the principle of health as a citizen's right and a state duty.3 Thus, Brazil's Ministry of Health (MoH) decided to provide access to DAAs and to update hepatitis C treatment in the country starting in 2014.4 From that date until December 2017, approximately 70,000 people received treatment with DAAs, at an approximate cost of US$ 1 billion.5, 6
In order to guarantee universal, unrestricted access to hepatitis C diagnosis and treatment and in line with WHO elimination goal, the MoH outlined a specific national strategy to achieve this target.1 This experience could be useful as a guide for other countries with similar epidemiologic, economic, or cultural characteristics. The objective of this paper was to describe the origins and outcomes of the Brazilian strategy including an analysis of the national hepatitis C disease burden and economic modeling of potential strategies for achieving WHO targets.
A mathematical modeling approach was used to estimate the current HCV-infected Brazilian population, to forecast future disease progression as well as to evaluate possible strategies and costs to eliminate HCV in Brazil by 2030.
The model
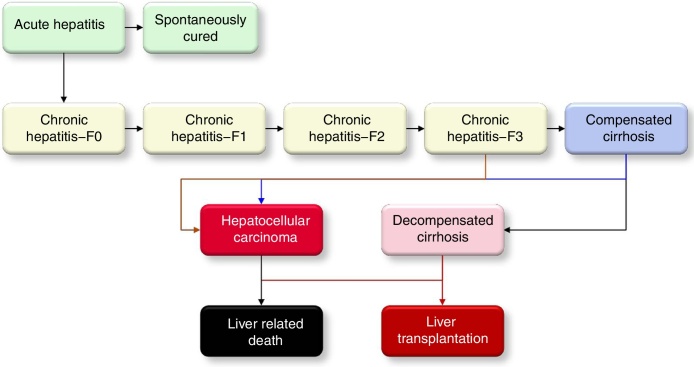
A disease progression Markov model, constructed in Microsoft Excel® (Microsoft Corp., Redmond, WA, USA), was utilized to quantify the size of the HCV-infected population, by the liver disease stages, from 1950 to 2030. The model is described in detail elsewhere.7 It was populated and calibrated using Brazilian specific epidemiologic data to forecast the HCV disease burden under different scenarios. The flow of patients in the Markov model is shown in Fig. 1.
Fig. 1.
The HCV disease progression in the Markov model.
Hepatitis C disease burden inputs
The historical epidemiology of HCV was gathered through a literature search, analysis of unpublished data and discussion with expert panels, as described previously.8 A review of the literature was conducted to identify indexed articles reporting epidemiology, age and sex distributions of HCV infection and total number of HCV cases diagnosed, treated and cured. The review encompassed all studies between January 1990 and March 2017. In addition, non-indexed sources were identified through the MoH and non-indexed journals. Finally, an expert panel provided proceedings of local conferences or unpublished data. Individuals were identified to participate based on contribution to prior published research on the subject, pertinent expertise and relevant responsibilities within the MoH. Two face-to-face meetings were conducted to review inputs, findings and analyses with the expert panel and incorporate their feedback.
When no input data were available, it was explicitly stated, and analogs (data from countries with a similar healthcare practice and/or risk factors) or expert inputs were used. The annual number of liver transplants was collected from the MoH database9 and adjusted for the percentage attributed to HCV.
HCV antibody prevalence and viremic prevalence
In 2016, 0.76% of 484,300 anti-HCV Ab rapid diagnostic tests conducted across Brazil among 15–69 year olds were antibody positive (unpublished data). After adjusting for regional populations and factoring in prevalence and the size of various at-risk groups (e.g., prisoners, people who inject drugs, crack users, men who have sex with men, and HIV-infected individuals), the anti-HCV prevalence among 15–69 years olds was estimated to be 0.70%.
The age and sex distribution were calculated using notification data from the MoH, which was published in the 2016 Boletim Epidemiologico report.10 The annual data from 2004 to 2016 were calibrated to the prevalence estimate and aged through the model to account for cured patients and mortality. Due to lack of data, it was assumed that the prevalence decreased by 50% with each 5-year age cohort for those older than 65–69 years and those younger than 15–19 years. Additionally, the anti-HCV prevalence among 15–24 year olds was calibrated to 0.18% to match the results from a large study (n = 36,818) of military conscripts.11
A viremic rate of 60.7% was calculated through a weighted average of five studies in Brazil.12, 13, 14, 15
Although a number of international studies report viremic rates, those results were influenced by the type of anti-HCV tests and the age of the population being diagnosed. For the purposes of this analysis, only studies in Brazil were considered.
Genotype distribution
The genotype distribution was provided by experts from national notification data.6
Incidence of new HCV infections
In this analysis, the term “incidence” refers to the absolute number of new infections occurring in a given year, rather than newly diagnosed cases. The incidence was back-calculated using the known prevalence as described previously.7 This analysis was supplemented by an analysis of HCV infection among people who inject drugs (PWID). There are an estimated 286,000 people who inject drugs (PWID) in Brazil, with an HCV seroprevalence of 26% corresponding to 73,000 HCV infections among PWID.16, 17 A turnover of 10% was used to estimate incidence among PWID. The prison population had no impact on the overall analysis. There are 700,000 prisoners with an estimated HCV prevalence of 13.6%.18 The majority of these infections are due to the PWID population, already captured above, as well as tattoos and high risk behavior in prisons.
Previously and annually diagnosed cases
Data on the number of newly diagnosed cases per year were provided by experts from the national, (unpublished) notification data. They were adjusted for under-reporting, mortality and treated cured to estimate the final number of diagnosed cases alive today and annual number of newly diagnosed. The analysis also took into consideration the number of individuals diagnosed through a national screening campaign. In 2017, a national campaign tested 6.1 million Brazilians via rapid testing and another 2.9 million via traditional serological testing. An estimated 30,000 cases were identified in this campaign.6
Number needed to be screened
The number of individuals that needed to be screened was calculated using the newly diagnosed rate from the historical screening campaign. It was assumed that future screenings will have a similar productivity. For screenings in high risk population, it was assumed that HCV prevalence will be five times higher in the high risk populations; thus, five time as many HCV positive cases will be identified.
Treated and cured patients
The expert panel also provided the number of treated patients in Brazil by year from national unpublished data (Table 3). According to expert consensus, the DAAs achieved a 95% SVR on average across all genotypes and disease stages.
Table 3.
DALY parameters.
| Age-weighting modulation constant (K) | 0 (no age-weighting) |
| Disability weight by disease stage | Used previously published estimates when available19 |
| F0–F4 | 0 |
| Decompensated cirrhosis | 0.178 |
| Hepatocellular carcinoma | 0.466a |
| Liver Transplant | 0.024b |
DALY, disability-adjusted life year.
Weighted average of disability weights for terminal and controlled phases of liver cancer due to hepatitis C. We assumed 85% of hepatocellular carcinoma cases were terminal (disability weight of 0.54) and 15% of cases were controlled (disability weight of 0.049).
Disability weight for end-stage renal disease, when kidney transplant was used.
Scenario analysis
Base scenario
We calculated the impact on the HCV infections and mortality if there is no change to HCV treatment policies from 2017 to 2030. In 2016, the following patients were eligible for treatment: patients staged ≥F3, patients with confirmed stage F2 in the previous three years, and patients with comorbidities such as HIV co-infection, chronic kidney disease or extra hepatic manifestations. Though there was a substantial increase in the number of patients treated between 2015 (∼14,000) and 2016 (∼37,000), the number of patients treated dropped to 23,000 in 2017. We assumed a continuing decline between 2018 and 2020 due to a shrinking pool of eligible patients. In 2017, national treatment guidelines were expanded to include all F2 patients. As from March 2018 treatment access was expanded to all infected patients.
National strategy plan scenario (NSP)
An intervention scenario was modeled to determine the degree to which the number of patients treated and diagnosed must increase starting in 2018 in order to reach the WHO targets by 2030.
Hepatitis C economic analysis
A modeling approach was used to evaluate the relative costs of two different strategies to control the HCV disease burden in Brazil: (1) if no further changes are made to the HCV treatment program in Brazil; (2) where the World Health Organization (WHO) targets for 2030 elimination are met by 2030. Data regarding direct costs were obtained from the Brazilian Unified Health System, and a Delphi process was applied in order to gain expert consensus and validate inputs. HCV-related costing data are detailed in Table 1, Table 2.
Table 1.
Diagnostic costs (2016).
| Cost per diagnosed and treated patient (US$) | # of tests for treating – present | # of tests for treating – 2022 | |
|---|---|---|---|
| Anti-HCV | 3 | 1 | 1 |
| RNA Test/PCR | 16 | 4 | 2 |
| Genotyping | 32 | 1 | 1 |
| Staging/Liver biopsy/fibroscan | 54 | 1 | 1 |
Table 2.
Healthcare costs (2016).
| Annual cost per diagnosed patient (US$) | |
|---|---|
| Annual follow-up F0–F2 | 185 |
| F3 to compensated cirrhosis | 219 |
| Decompensated cirrhosis | 3340 |
| Hepatocellular carcinoma | 5886 |
| liver transplant | 38,202 |
| Liver transplant – subsequent years | 2385 |
The economic analysis factored in direct costs of screening, diagnosing and treating HCV; healthcare costs associated with HCV and advanced liver disease; and indirect costs to the society from years of life lost (YLL) and years lived with disability (YLD) due to HCV-related morbidity and mortality.
Average cost of DAAs (across genotypes) for a 12-week course of treatment was estimated at US$ 4600 in 2017 and was estimated to decreased to US$ 2700 by 2019 per expert input.
Direct costs
Direct costs were those associated with screening, diagnosing and managing chronic HCV infection, cirrhosis and liver cancer (in the absence of antiviral therapy) and treating HCV.
Indirect costs
Disability-adjusted life years (DALYs) were calculated based on years lived with disability (YLDs) weighted by disease stage (minimum disability impact until cirrhosis, liver cancer or transplant) and YLLs. Weighting of disability by disease stage was taken from the health literature as shown in Table 3. Disability weights were applied only to diagnosed cases for F0–F3 and for all prevalent cases in F4 and advanced disease stages.
Indirect costs were taken as economic productivity losses to Brazilian society caused by HCV and related liver disease and mortality. We assumed the value of a statistical life year (VSLY) was equal to the gross national income (GNI) per capita in Brazil, US$ 7772 in 2015 (from the World Bank 2015). Indirect costs were calculated only for DALYs incurred among cases aged 20–69 years
Results
Hepatitis C disease burden
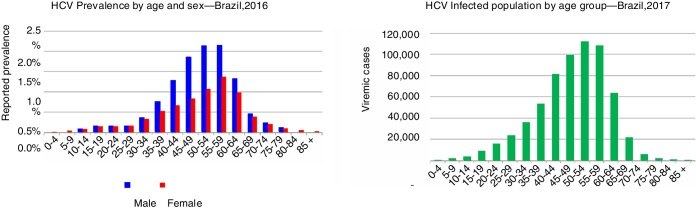
After taking into consideration all ages, an anti-HCV prevalence of 0.53% was calculated for the total population (0–85+) corresponding to 1,091,000 antibody positive cases in 2016. It was estimated that the number of HCV-RNA+ individuals in Brazil in 2017 was 632,000 (0.31% of the population). The prevalence and total infections by age are shown in Fig. 2.
Fig. 2.
Hepatitis C prevalence by age and sex.
Genotype 1 accounted for 71.3% of all cases, followed by Genotype 3, which accounted for 24.4% of cases.
The back-calculations of incidence estimated 7500 new acute HCV infections in 2016. This was consistent with estimating new infections among PWID. Assuming a turnover of 10% of HCV infections among PWID, an incidence of 7300 per year was estimated among this population.
The total reported diagnosed cases were 319,000 antibody positive cases in 1999–2016. After adjusting for under-reporting, an estimated 362,000 antibody positive cases were diagnosed in the same period. The annual data were then adjusted for mortality and cured cases, resulting in an estimated 224,000 antibody positive diagnosed cases who are alive today. Treated and cured cases were among the viremic population only. Thus, it was estimated that 97,000 viremic diagnosed cases (not cured and alive) remained in 2016. The estimated number of newly diagnosed viremic cases in 2016, accounting for under-reporting, was estimated at 18,800. A summary of the model inputs is shown in Table 4.
Table 4.
Epidemiologic data used as inputs in the model.
| Input | Estimate year | |
|---|---|---|
| RNA+HCV infections | 662,000 | 2016 |
| Total diagnosed (RNA+) | 97,000 | 2016 |
| Annual newly diagnosed | 30,000 | 2017 |
| Annual number treated | 23,000 | 2017 |
Scenario analysis
The numbers of people who need to be screened, diagnosed and treated under each scenario are shown in Table 5. The number of rapid tests that will be needed to meet the diagnosis target was calculated for each year with the assumption that screening continues to find positive cases at the current positivity rate (0.55% anti-HCV). In order to achieve the WHO targets by 2030, treatment access was expanded to all fibrosis stages beginning in 2018. Patients aged 15–79 continued to be eligible for treatment. The number of treated patients increased to a peak of 50,000 in 2019–2020, and the number of annually diagnosed patients increased to a maximum of 40,000 starting in 2019.
Table 5.
The number of people who need to be screened, diagnosed and treated for each scenario.
| 2016 | 2017 | 2018 | 2019 | 2020 | 2025 | |
|---|---|---|---|---|---|---|
| Base | ||||||
| Treated | 36,600 | 23,000 | 19,000 | 12,900 | 12,500 | 12,500 |
| Newly diagnosed | 18,800 | 30,000 | 30,000 | 30,000 | 30,000 | 30,000 |
| Number needed to screen (gen. pop.) | 3,889,000 | 9,000,000 | 9,585,000 | 10,246,000 | 10,991,000 | 16,763,000 |
| Number needed to screen (high risk. pop.) | 778,000 | 1,800,000 | 1,917,000 | 2,049,000 | 2,198,000 | 3,353,000 |
| NSP | ||||||
| Treated | 36,600 | 23,000 | 19,000 | 50,000 | 50,000 | 32,000 |
| Newly diagnosed | 18,800 | 30,000 | 30,000 | 40,000 | 40,000 | 40,000 |
| Number needed to screen (gen. pop.) | 3,889,000 | 9,000,000 | 9,598,000 | 13,975,000 | 15,469,000 | 30,997,000 |
| Number needed to screen (high risk. pop.) | 780,000 | 1,800,000 | 1,920,000 | 2,795,000 | 3,094,000 | 6,199,000 |
NSP, national strategy plan.
The screening strategy would be more efficient if testing targeted high risk populations. Table 5 shows the number of people who need to be screened if the screening campaign targeted high prevalence populations (defined as having five-fold higher prevalence than the current rate) for the same strategies considered above.
As shown in Table 6, the analysis projected that in the Base Scenario the total number of HCV infections would decline from 2015 to 2030, but HCC would increase by 25% and decompensated cirrhosis would be 45% higher than in 2015 as the population ages. The number of liver related deaths would be 30% higher in 2030 than in 2015. Compared with the base case, the NSP scenario drastically reduces hepatocarcinoma, decompensated cirrhosis and liver related deaths.
Table 6.
Projected prevalence, morbidity and mortality in each scenario in 2020 and 2030.
| Prevalent viremic cases | Incident HCC | Incident decompensated cirrhosis | Incident liver related deaths | |
|---|---|---|---|---|
| Base case | ||||
| 2020 | 607,000 | 2500 | 1900 | 2500 |
| 2030 | 469,000 | 2800 | 2200 | 3600 |
| NSP | ||||
| 2020 | 531,000 | 2400 | 1800 | 2300 |
| 2030 | 125,000 | 910 | 730 | 970 |
Economic analysis
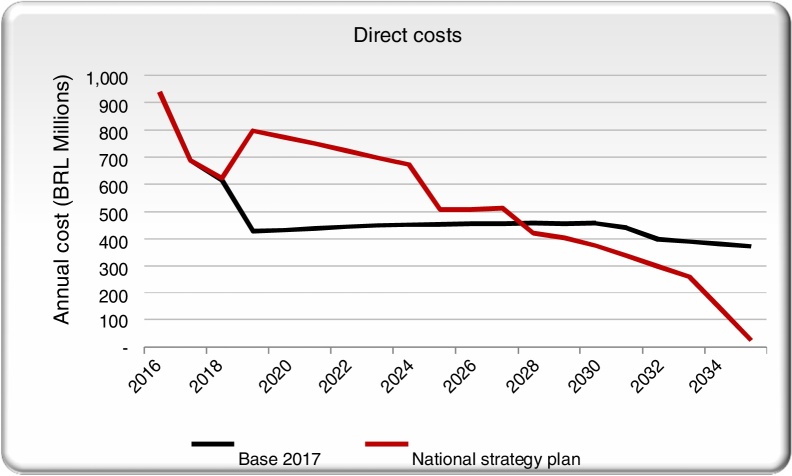
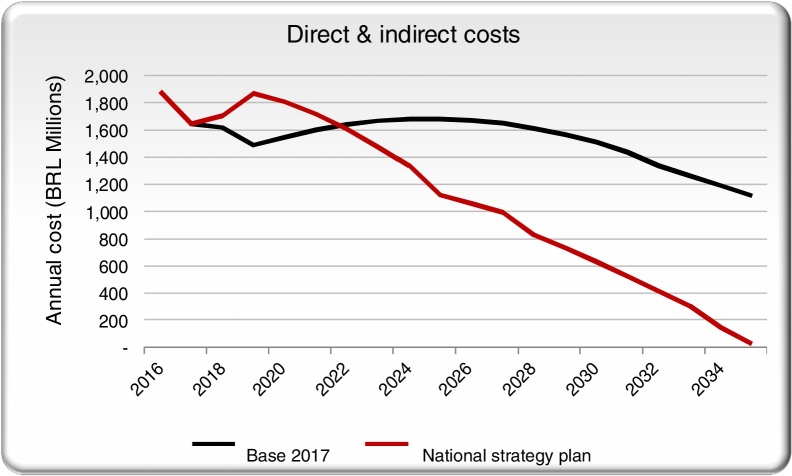
We aggregated the direct costs for the base case and the NSP scenario and compared them across time. Screening, diagnostic and treatment annual costs are projected to increase significantly with the scale-up of treatment and diagnosis in the initial years of the NSP scenario, but then drop below the base case on an annual basis by 2035, once HCV is eliminated (Fig. 3).
Fig. 3.
Direct costs in the NSP scenario and the base case scenarios.
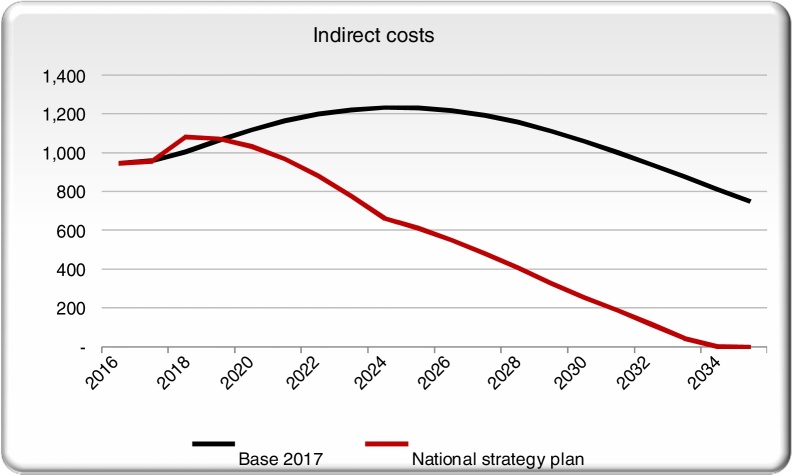
Indirect costs in the NSP scenario remain approximately even with the base case for the first couple years before dropping (Fig. 4). As more patients receive treatment and are cured, reduced mortality and disability will result in fewer YLLs and YLDs. As a result, DALYs are avoided in the NSP scenario, creating savings in indirect costs.
Fig. 4.
Indirect costs in the NSP scenario and the base case scenarios.
The 20-year average cost per DALY averted was US$ 486 in the NSP scenario, well under the GNI per capita of US$ 7838, indicating cost-effectiveness.
We also calculated the annual total cost (direct + indirect costs) to Brazil and found the NSP scenario will result in a lower cost, relative to the base scenario, starting in 2022 (Fig. 5).
Fig. 5.
Comparing direct and indirect costs in the NSP and base scenarios.
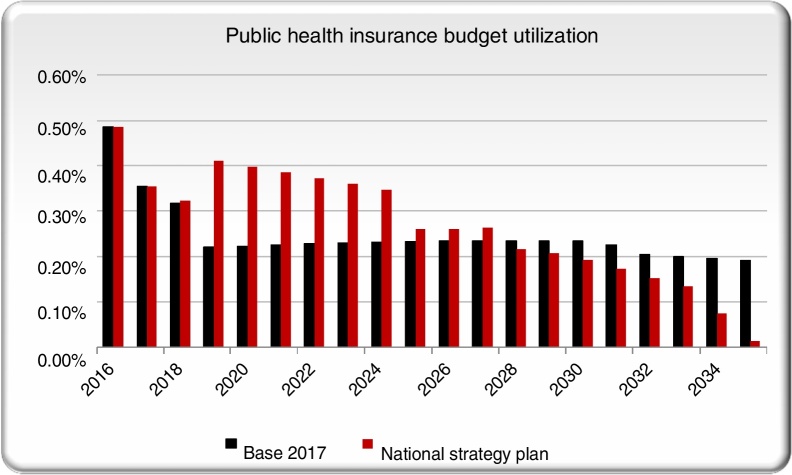
Additionally, the cumulative (2017 forward) direct and indirect costs to Brazilian society under the NSP scenario was cost saving with a positive return on investment (ROI) starting in 2025. The proportion of the total public health budget (estimated in US$ 53 billion) spent on HCV management is projected to decline in future years in the base scenario as the number of treated patients declines (Fig. 6). As a result, the NSP scenario is expected to require a larger percentage of the total public health budget initially. After 2028, the NSP scenario will cost less than the base scenario and after 2030 it will require less than 0.2% of the budget as the elimination targets are met and there are fewer patients with advanced liver disease.
Fig. 6.
Comparing public health budget utilization in the NSP scenario with the base case scenario.
Discussion
Hepatitis C elimination plan in Brazil
The Ministry of Health of Brazil, in line with the WHO goal to eliminate hepatitis C as a global health threat by 2030, has outlined a national strategy to achieve this target. A mathematical modeling approach was used to estimate the current HCV-infected Brazilian population, to project future disease progression including liver cancer and deaths, and to evaluate possible strategies and costs to eliminate HCV in Brazil by 2030. In 2016, it was estimated that the anti-HCV prevalence was 0.53% among the total population and that the number of HCV-RNA+ individuals was 632,000 (0.31% of the population). It was also possible to project that if no change were made to Brazil's HCV treatment strategy (although over 70,000 Brazilian patients had been treated with new DAAs as of December 2017), there would be an increase in cases of liver cancer and advanced liver disease by 2030.
According to the modeled intervention, it would be necessary to combine an increase of treatment and diagnosis over time in order to reach WHO targets by 2030. Another insight that the modeling provided was that WHO targets would require significant up-front investment in treatment and diagnosis. However, savings from healthcare and indirect costs as the disease burden is reduced would offset these costs, resulting in lower total annual costs by 2022 and a positive return on investment (ROI) by 2025 when compared against the Base Scenario.
The results of this study allowed the MoH's Department of STIs, HIV/AIDS and Viral Hepatitis to outline the Hepatitis C Elimination Plan in Brazil, which was then approved by the MoH's Tripartite Commission (represented by the Brazilian federal, state and municipal governments) in October 2017. Since then, the MoH's Department of STIs, HIV/AIDS and Viral Hepatitis has been ensuring the feasibility of this plan, establishing a universal treatment policy for all viremic cases, updating therapeutic guidelines, simplifying tests and implementing interventions for linkage to care, and establishing a sustainable price negotiation policy over time.
Hepatitis C disease burden in Brazil
The estimates assumed following the construction of this model demonstrate a significantly lower HCV prevalence when compared to a previous study conducted from 2005 to 2009 in a representative sample of 19,503 adolescents and adults in all Brazilian macro-regions. That study demonstrated an anti-HCV Ab prevalence of 1.38%,15 a prevalence not since replicated in other studies. Additionally, the published viremic rate of 35.7% suggests a relatively high HCV Ab false positivity rate. It is also possible that the reduction of seroprevalence observed in the present study reflects a change in the pattern of HCV transmission in Brazil over the past 20 years.
Before blood screening began in 1992 in Brazil, transfusion of blood and blood products was the predominant route of HCV transmission.20 The model estimates that more than 90% of the infected population was born prior to blood screening. Injection drug use has also been an important mode of hepatitis C transmission in the past.20, 21 Today blood and blood products transfusions are safe.22 In addition, a significant reduction in the overall frequencies of drug injection and needle-sharing has been observed in the country.17, 23 All these factors could have contributed to a reduction in hepatitis C transmission and viremic cases. Nevertheless, nosocomial transmission (particularly hemodialysis), as well as transmission through needle sharing for therapeutic injections in nonmedical settings, could contribute to ongoing hepatitis C transmission in the country.24, 25
In order to prevent the spread of HCV in dialysis units, MoH has established specific guidelines. Despite these guidelines, patients on hemodialysis treatment are still at high risk for HCV infection.23, 24 In Brazil, the risk of exposure to HCV has also been associated with the practice of tattooing and body piercing without attention to sterilization or use of disposable equipment as well as with sex behavior.21 However, the frequency of these events seems to be low and with a lower impact on the overall infections.1 Our data estimate that the majority of patients with hepatitis C in Brazil are aged between 40 and 65. These data reinforce the need for a priority diagnosis in people in this age group.
Hepatitis C economic analysis
The model outputs revealed that the current Brazilian strategy to confront this epidemic can be made significantly more cost-effective while accelerating the elimination of the disease burden, and thus modification is urgently needed. From 2005 to 2015 an extraordinary increase of MoH expenditure on medicines for hepatitis C was observed, mainly due to an increase of volumes purchased as well as the need to incorporate alfa-pegylated interferon in the early DAA combinations.26 In 2015 the adoption of the new DAA led to an increase of 230% (US$ 255 million) in MoH spending, as compared to 2014.26 The current Brazilian strategy, despite its huge investment, would not be able to guarantee in the medium and long term the elimination of this disease. On the contrary, maintaining the same type of public health policy could lead to a significant increase in the number of advanced liver disease cases and the need for further increased investment.
Based on our estimates, achieving the WHO targets demands a scale-up of treatment and diagnosis over time, beginning in 2019. A major challenge will be to sustain strategic actions and to increase the number of newly diagnosed patients in order to maintain a pool of patients who are eligible for treatment to reduce the size of the epidemic.
The elimination scenario projects that antiviral will constitute a substantial proportion of public health expenditure to address HCV. Any further reductions in DAA prices from those assumed in the model will improve cost-effectiveness and ultimately reduce budget impact, strengthening the economic case for elimination. The critical nature of achieving affordable DAA pricing for the nation cannot be underestimated.
It is important to note that the results of this study are influenced by several limitations inherent to mathematical modeling. Inputs used in the model, concerning the epidemiology of hepatitis C in Brazil, were not always published or available in the literature. To deal with this, the authors applied the Delphi process, relying on a panel of experts whenever the information was not based on peer-reviewed published literature. Secondly, we have assumed that the number of new cases would remain constant into the future. This is a conservative approach that assumes static trends in risk behavior in the absence of more information. Thirdly, we have assumed that screening campaigns will continue to deliver the same rate of newly diagnosed cases. In reality, it is possible that as the diagnosis rate increases, it will become more difficult to find undiagnosed patients. Additionally, we have assumed a continuous availability of DAAs to diagnosed patients. In reality, challenges associated with price negotiation and drug distribution may prevent diagnosed patients from accessing medication as predicted by the model. Finally, the model does not take into consideration the possible impact, on either HCV disease burden or on the HCV economic analysis, of the progression of cured HCV patients, reinfection, comorbidities and extrahepatic manifestations of HCV infection. These could lead to increased future healthcare costs. However, these limitations are typical of similar studies and we believe they do not significantly compromise the relevance and magnitude of the results presented.
Conclusion
In conclusion, our study indicates that elimination of hepatitis C in Brazil is technically feasible, and HCV disease burden would not be controlled by the previous treatment strategy. However, the elimination of hepatitis C requires policy changes to substantially scale-up prevention, screening, and treatment of HCV, together with public health advocacy to raise awareness among affected populations and healthcare providers. Healthcare providers, including primary healthcare practitioners, will need specific training in the diagnosis and treatment of HCV. Testing and counseling must be scaled up and also directed at prioritized groups with higher HCV prevalence including HIV infected patients, diabetics, adults over 40 years, or patients on hemodialysis. Given these specific populations are already engaged with the nation's Unified Health System (SUS), they could be easily identified and referred for HCV treatment.
Government, medical societies and industry need now to work together in order to assure full access to the new antiviral regimens and health services for everyone who is infected. Access must not be compromised by excessively high prices or lack of political will. Elimination of hepatitis C in Brazil is possible but will require urgent, strong and sustained political and societal commitment to achieve this goal.
Funding
The mathematical model was funded by The Ministry of Health in Brazil.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Boletim epidemiológico hepatites viraism, vol. 49, no. 31-2018. Brasília: Ministério da Saúde, Secretaria de Vigilância em Saúde.
- 2.World Health Organization . World Health Organization; 2016. Combating hepatitis B and C to reach elimination by 2030: advocacy brief. http://www.who.int/iris/handle/10665/206453. [Google Scholar]
- 3.Moura Elisângela Santos de. O direito à saúde na Constituição Federal de 1988. In: Âmbito Jurídico, Rio Grande, XVI, n. 114, jul 2013. Disponível em:.http://www.ambitojuridico.com.br/site/?n_link=revista_artigos_leitura&artigo_id=13440. Acesso em jan 2019.
- 4.Mesquita F., Santos M.E., Benzaken A., et al. The Brazilian comprehensive response to hepatitis C: from strategic thinking to access to interferon-free therapy. BMC Public Health. 2016;16:1132. doi: 10.1186/s12889-016-3784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaves G.C., Castro C.G.S.O., Oliveira M.A. Public procurement of hepatitis C medicines in Brazil from 2005 to 2015. Ciên Saúd Col. 2017;22:2527–2538. doi: 10.1590/1413-81232017228.05602017. [DOI] [PubMed] [Google Scholar]
- 6.Brasil. Ministério da Saúde. DAF/DIAHV. 2017
- 7.Blach S., Zeuzem S., Manns M., et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 8.Bruggmann P., Berg C., Øvrehus A., et al. Historical epidemiology of hepatitis C virus (HCV) in select countries. J Viral Hepat. 2014;21:5–33. doi: 10.1111/jvh.12247. [DOI] [PubMed] [Google Scholar]
- 9.Bittencourt P.L., Farias A.Q., Couto C.A. Liver transplantation in Brazil. Liver Transpl. 2016;22:1254–1258. doi: 10.1002/lt.24487. [DOI] [PubMed] [Google Scholar]
- 10.Boletim epidemiológico hepatites virais. Brasília: Ministério da Saúde, Secretaria de Vigilância em Saúde, Ano 5, No. 1-2016.
- 11.Brasil. Ministério da Saude. 2018. Departamento das IST, do HIV/Aids e das Hepatites Virais. Estudo Epidemiológico sobre a prevalência da infecção por sífilis, HIV, hepatites virais B e C e dos fatores comportamentais associados em conscritos das forças armadas. 8@ Edição.
- 12.Puga M.A.M., Bandeira L.M., Pompilio M.A., et al. Prevalence and incidence of HCV infection among prisoners in Central Brazil. PLOS ONE. 2017;12:e0169195. doi: 10.1371/journal.pone.0169195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costi C., Grandi T., Halon M.L., et al. Prevalence of hepatitis C virus and human immunodeficiency virus in a group of patients newly diagnosed with active tuberculosis in Porto Alegre Southern Brazil. Mem Inst Oswaldo Cruz. 2017;112:255–259. doi: 10.1590/0074-02760160352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto F.P., Ferreira O.C., Jr., Olmedo D.B., et al. Prevalence of hepatitis B and C markers in a population of an urban university in Rio de Janeiro, Brazil: a cross-sectional study. Ann Hepatol. 2015;14:815–825. doi: 10.5604/16652681.1171756. [DOI] [PubMed] [Google Scholar]
- 15.Pereira L.M., Martelli C.M., Moreira R.C., et al. Prevalence and risk factors of Hepatitis C virus infection in Brazil, 2005 through 2009: a cross-sectional study. BMC Infect Dis. 2013;13:60. doi: 10.1186/1471-2334-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva M.B., Andrade T.M., Silva L.K., et al. Prevalence and genotypes of hepatitis C virus among injecting drug users from Salvador-BA, Brazil. Mem Inst Oswaldo Cruz. 2010;105:299–303. doi: 10.1590/s0074-02762010000300009. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira M.L., Yoshida C.F., Telles P.R., et al. Trends in HCV prevalence, risk factors and distribution of viral genotypes in injecting drug users: findings from two cross-sectional studies. Epidemiol Infect. 2009;137:970–979. doi: 10.1017/S0950268808001970. [DOI] [PubMed] [Google Scholar]
- 18.Magri M.C., Ibrahim K.Y., Pinto W.P., França F.O.S., Bernardo W.M., Tengan F.M. Prevalence of hepatitis C virus in Brazil's inmate population: a systematic review. Rev Saúde Públ. 2015;49:42. doi: 10.1590/S0034-8910.2015049005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abajobir A.A., Abate K.H., Abbafati C., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study. Lancet. 2016;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toledo A.C., Jr., Greco D.B., Felga M., Barreira D., Gadelha M.F., Speranza F.A. Seroprevalence of hepatitis B and C in Brazilian army conscripts in 2002: a cross-sectional study. Braz J Infect Dis. 2005;9:374–383. doi: 10.1590/s1413-86702005000500004. [DOI] [PubMed] [Google Scholar]
- 21.Kretzer I.F., Livramento A., Cunha J., et al. Hepatitis C worldwide and in Brazil: silent epidemic – data on disease including incidence, transmission, prevention, and treatment. Scient World J. 2014:827849. doi: 10.1155/2014/827849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agência Nacional de Vigilância Sanitária|ANVISA; 2017. Brasil. 4° Boletim de Produção Hemoterápica Hemoprod 2014 e 2015. [Google Scholar]
- 23.Guimarães M.L., Marques B.C., Bertoni N., et al. Assessing the HIV-1 epidemic in brazilian drug users: a molecular epidemiology approach. PLoS ONE. 2015 doi: 10.1371/journal.pone.0141372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho-Filho R.J., Feldner A.C., Silva A.E., Ferraz M.L. Management of hepatitis C in patients with chronic kidney disease. World J Gastroenterol. 2015;21:408–422. doi: 10.3748/wjg.v21.i2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva N.M., Germano F.N., Mendoza-Sassi R.A., Seuánez H.N., Soares M.A., de Martinez A.M. Evidence of association between hepatitis C virus genotype 2b and nosocomial transmissions in hemodialysis centers from southern Brazil. Virol J. 2013;10:167. doi: 10.1186/1743-422X-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaves G.C., Castro C.G.S.O., Oliveira M.A. Public procurement of hepatitis C medicines in Brazil from 2005 to 2015. Cien Saude Colet. 2017;22:2527–2538. doi: 10.1590/1413-81232017228.05602017. [DOI] [PubMed] [Google Scholar]