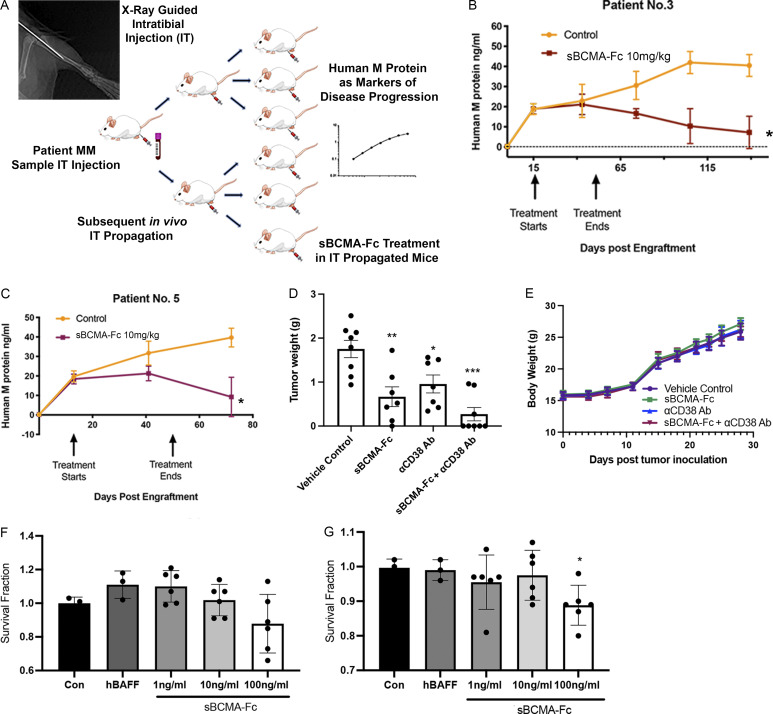
Figure S4.
Wild-type sBCMA decoy receptor inhibits MM growth in vivo but lacks efficacy in BAFF-driven DLBCL. (A) Schematic flowchart of in vivo MM PDX propagation. Patient tumor cells were isolated from patient bone marrow biopsies and injected into the tibias of NSG mice. PDX were subsequently propagated in vivo using the same intratibial inoculation procedure and treated with vehicle control or sBCMA-Fc. Human IgG protein in mouse serum was continuously monitored over time as a marker of tumor progression. (B) Human IgG protein in mouse serum detected in animals successfully engrafted with MM cells from patient 3, showing reduction in human M protein level after sBCMA-Fc treatment (n = 8) compared with vehicle control (n = 7); P = 0.012. (C) Human IgG protein in mouse serum detected in animals successfully engrafted with MM cells from patient 5, showing reduction in human IgG protein level after sBCMA-Fc treatment (n = 10) compared with vehicle control (n = 10); P = 0.026. (D) Terminal tumor weight of MM1.R MM cells in mice dosed with sBCMA-Fc 10 mg/kg every 48 h (n = 7; P = 0.0078), αCD38 10 mg/kg weekly (n = 7; P = 0.01), sBCMA-Fc and αCD38 combination (n = 8; P < 0.0001), and vehicle control (n = 8) in 6-wk-old female NSG mice. (E) Changes in body weight of animals from study described in D. (F) sBCMA-Fc dose-dependent cytotoxicity assay validating in vitro cell survival in the presence of increasing doses of sBCMA-Fc and hBAFF (100 ng/ml) in SU-DHL-6 DLBCL cells. (G) sBCMA-Fc dose-dependent cytotoxicity assay validating in vitro cell survival in the presence of increasing doses of sBCMA-Fc and hBAFF (100 ng/ml) in Daudi DLBCL cells. Treatment with 100 ng/ml sBCMA-Fc led to significant reduction in cell number; P = 0.045. Statistical analysis was conducted using t test and one-way ANOVA for comparing between treatment groups. Repeated ANOVA used for changes in tumor growth over time. *, P < 0.05; **, P < 0.01; ***, P < 0.001.