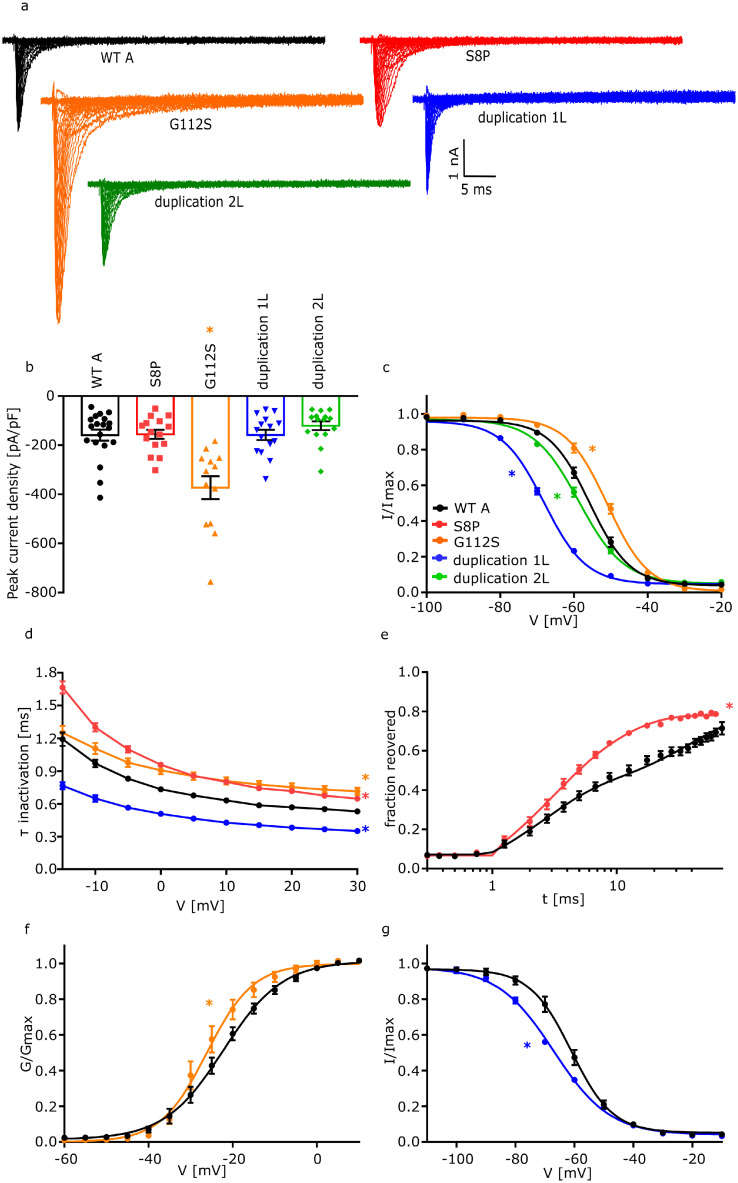
Figure 3.
Electrophysiological analysis of FGF12 variants on NaV1.6. Functional analysis of the effect of two FGF12 missense variants (S8P and G112S) and two FGF12 CNVs (duplication 1L and duplication 2L) on NaV1.6 compared to WTA. Statistically significant effects for each condition are indicated by coloured asterisks.
a. Representative traces of NaV1.6 currents in ND7/23 cells expressing NaV1.6 with FGF12 WTA or the FGF12 variants, respectively, in response to voltage steps from -80 to +35 mV in 5 mV steps.
b. Mean Current amplitudes of analysed ND7/23 cells injected with NaV1.6 with WTA (n=19; 8 transfections), S8P (n=15; 4 transfections; p = >0·999), G112S (n=13; 5 transfections; p = 0·0004), duplication 1L (n=15; 7 transfections; p = >0·999) or duplication 2L (n=15; 6 transfections; p = 0·999). Expression of NaV1.6 together with G112S showed a statistically significantly elevated current density compared to NaV1.6 with WTA. For the statistical analysis ANOVA on ranks with Dunn´s post hoc test was used.
c. Mean voltage-dependent fast inactivation of NaV1.6 with FGF12 WTA (n=16; 6 transfections), G112S (n=10; 3 transfections; p (V1/2) = 0·04), duplication 1L (n=12; 5 transfections; p (V1/2) = <0·0001) or duplication 2L (n=11; 5 transfections; p (k) = 0·0003). Lines illustrate Boltzmann Function fit to the data points. G112S showed a statistically significant shift to more depolarized potentials in comparison to WTA while both Duplications show a hyperpolarizing shift in comparison to WTA. For the statistical analysis ANOVA on ranks with Dunn´s post hoc test was used. All data are shown as means ± SEM.
d. Mean voltage-dependent fast inactivation time constant of NaV1.6 FGF12 WTA (n=19; 8 transfections), S8P (n=15; 4 transfections; p = 0·001), G112S (n=13; 5 transfections; p = 0·05) or duplication 1L (n=15; 7 transfections; p = 0·002). S8P and G112S show a statistically significantly slowed fast inactivation in comparison to WTA while duplication 1L shows an accelerated fast inactivation. For the statistical analysis ANOVA on ranks with Dunn´s post hoc test was used. All data are shown as means ± SEM.
e. Time course of recovery from fast inactivation of NaV1.6 with FGF12 WTA (n=10; 8 transfections) or FGF12 S8P (n=11; 4 transfections; p (τ2) = <0·0001) determined at -100 mV. The variant S8P leads to a statistically significantly accelerated recovery of fast inactivation in comparison to WTA. Lines represent fits of biexponential functions yielding the time constants τ1 and τ2. A1 was set to 0·3. For the statistical analysis ANOVA on ranks with Dunn´s post hoc test was used.
f. Mean voltage-dependent activation of NaV1.6 with FGF12 WTA (n=19; 8 transfections) or G112S (n=13; 5 transfections; p (k) = 0·04). G112S shows a hyperpolarizing shift in comparison to WTA. Lines illustrate Boltzmann Function fit to the data points. For the statistical analysis ANOVA on ranks with Dunn´s post hoc test was used.
g. Mean voltage-dependent slow inactivation of NaV1.6 with FGF12 WTA (n=11; 8 transfections) or FGF12 duplication 1L (n=10; 5 transfections; p (V1/2) = 0·002). Lines illustrate Boltzmann Function fit to the data points. FGF12 duplication 1L shows a shift to more hyperpolarized potentials in comparison to NaV1.6 with FGF12 WTA. For the statistical analysis of the slow inactivation one-way ANOVA with Holm-Sidak´s post hoc test was used. All data are shown as means ± SEM. All values of electrophysiological results, numbers and p-values are listed in Table 1 and are shown as means with the 95% confidence interval.