Figure II in Box 3. Integration-induced Immune Evasion.
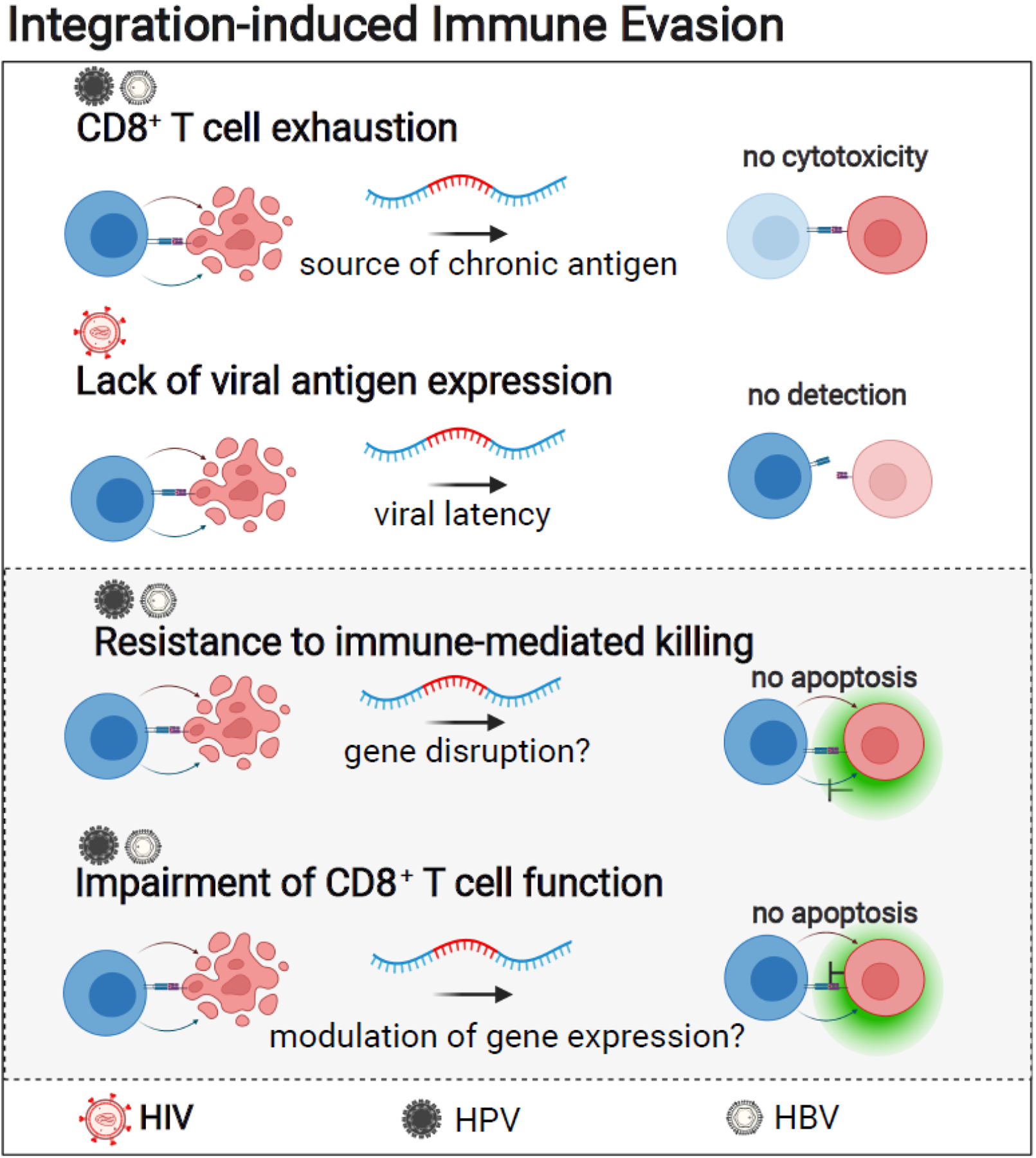
Genomic integration of HPV and HBV can target genes that control cellular functions related to immune evasion. Viruses for which experimental evidence exists for integration and transcriptional upregulation of genes involved in the indicated cellular functions are listed above each cellular mechanism. HBV, HPV, and EBV integration can upregulate the expression of anti-apoptotic proteins [34,35,65–67]. HBV integration can result in upregulation of FOXP2, a gene that encodes a transcription factor with anti-apoptotic functions [66,67]. Anti-apoptotic genes targeted by HPV include ETS2 [34] as well as BABAM2 [35], a gene that encodes an anti-apoptotic protein that blocks tumor necrosis factor alpha (TNF-α)-induced apoptosis [95]. Likewise, EBV integration can result in upregulation of an inhibitor of TNF-α-dependent apoptosis encoded by TNFAIP3 [65]. For HPV integration, an additional mechanism of immune evasion has been described; integration into CD274 results in upregulated encoded T-cell inhibitory receptor PD-L1 [34,35]. Expression of this inhibitory ligand functions as a generally recognized tumor immune escape mechanism that limits the proliferative and cytotoxic activity of cytotoxic T lymphocytes [97]. In addition to changes in host gene expression, changes in viral gene expression can contribute to immune evasion. Integration into less accessible chromatin regions might silence HIV-1 provirus and preclude antigen-presentation of viral epitopes. By contrast, integration and high amounts of viral protein expression of HBV sequences have been suggested to contribute to immune evasion by promoting T cell exhaustion of HBV-specific CD8+ T cells [98]. This figure was created with BioRender.com.
