FIG 1.
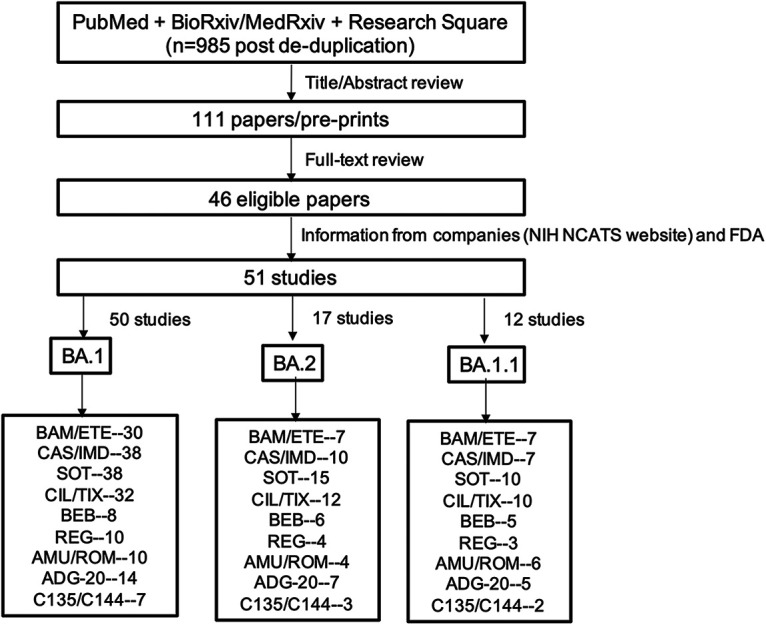
Flow chart of study selection process. Of 985 deduplicated studies identified through a search of PubMed and three preprint servers using the search string “SARS-CoV-2 AND Omicron AND (Neutralization OR Antibody OR Treatment),” 111 were read in their entirety following an initial review of titles and abstracts. Forty-six studies met our inclusion criteria in that they contained neutralizing susceptibility data for one or more FDA-authorized monoclonal antibodies (MAbs). Five additional data sets, including three FDA fact sheets and two data sets available on the NIH NCATs website, were also included. The number of studies containing susceptibility data for the Omicron BA.1 and BA.2 variants and the number of studies for each of the clinical-stage MAbs are shown. BAM, bamlanivimab; ETE, etesevimab; CAS, casirivimab; IMD, imdevimab; SOT, sotrovimab; CIL, cilgavimab; TIX, tixagevimab; REG, regdanvimab; ADI, adintrevimab; BEB, bebtelovimab; AMU, amubarvimab; ROM, romlusevimab. The presence of two MAbs separated by “/” indicates the combination was tested and/or that each individual MAb in the combination was also tested.
