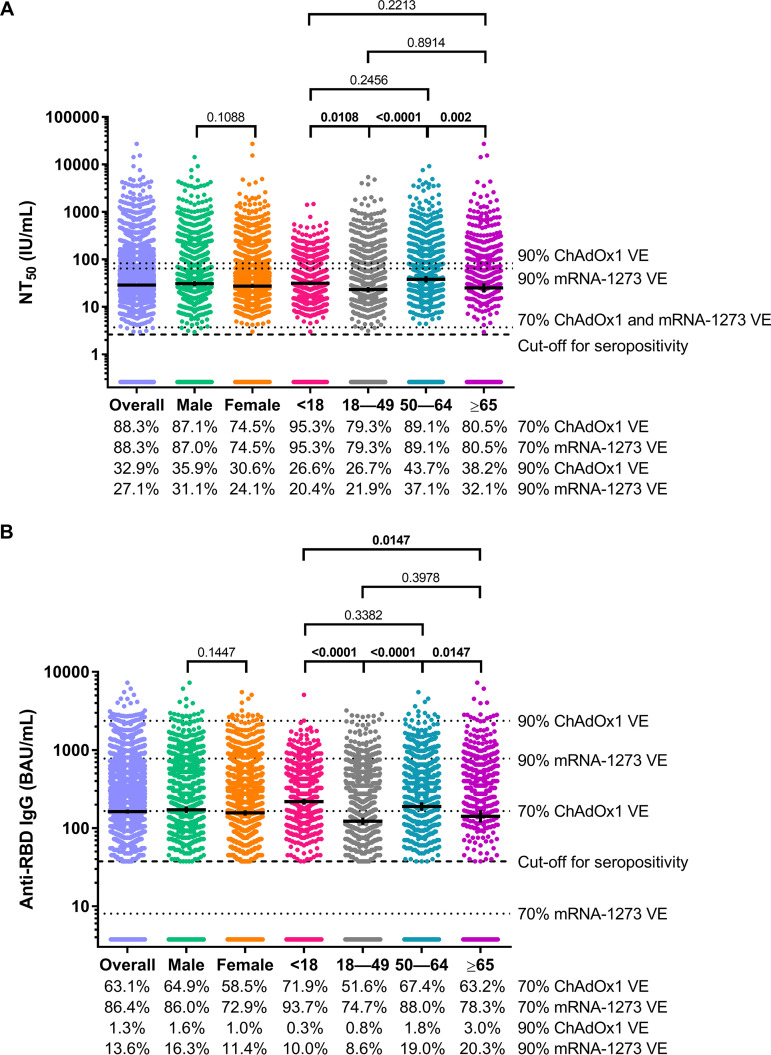
FIG 1.
SARS-CoV-2 50% neutralizing antibody titer (NT50) concentrations and anti-SARS-CoV-2 receptor binding domain (RBD) IgG concentrations for the overall convenience sample of 3,067 serum specimens collected during 27 July 2020 to 27 August 2020 with detectable anti-SARS-CoV-2 antibodies on a qualitative assay and according to sex and age category. SARS-CoV-2 NT50 concentrations in international units per milliliter (IU/mL) (A) and anti-SARS-CoV-2 RBD IgG concentrations in binding antibody units per milliliter (BAU/mL) (B) are shown. Horizontal bars represent geometric means, vertical error bars represent 95% confidence intervals, and dashed horizontal lines represent assay cutoff values for seropositivity. P values from t tests (for sex) and post hoc Tukey tests (for age class) are shown for each sex and age class comparison. Bolded P values denote statistical significance (P < 0.05). Dotted horizontal lines represent antibody concentrations associated with 70% and 90% ChAdOx1 (11) and mRNA-1273 (12) vaccine efficacy (VE). The percentage of sera with antibody concentrations that met or exceeded the concentrations represented by each of the horizontal dotted lines are shown below the charts.