ABSTRACT
In this study, we aimed to investigate the occurrence and molecular characteristics of fosfomycin-resistant Enterobacteriaceae isolates from pig, chicken and pigeon farms in Guangxi Province of China. A total of 200 fosfomycin-resistant strains were obtained from food animals and their surrounding environments, with the fosA, fosA3, and fosA7.5 genes being detected in 26% (52/200), 10% (20/200), and 5% (10/200), respectively. Surprisingly, three fosA7.5-producing E. coli isolates were found to be concomitant with fosA3. Most of the fosA-like-gene-positive isolates were multidrug-resistant strains and consistently possessed blaCTX-M-1/CTX-M-9, floR, and blaTEM genes. Only fosA3 was successfully transferred to the recipient strains, and the 29 fosA3-carrying transconjugants exhibited high-level resistance to fosfomycin (MIC ≥ 512 μg/mL). Multilocus sequence typing (MLST) combined with enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) analyses indicated that fosA3 or fosA7.5 genes were spread by horizontal transfer as well as via clonal transmission between E. coli. We used the PCR mapping method to explore the genetic contexts of fosA-like genes, and two representative strains (fEc.1 and fEcg99-1) were fully sequenced. Six different genetic structures surrounding fosA3 were detected and one infrequent context was discovered among the conjugable fosA3-positive E. coli isolates. The five genetic environments of fosA were identified and found to be highly similar to the partial sequence of transposon Tn2921. Furthermore, whole-genome sequencing (WGS) results showed that fosA7.5 was colocalized with mcr-3, blaCMY-63, sul3, tet(A), dfrA, and a number of virulence-related factors on the same chromosomes of strains, and various insertion sequences (IS3/ISL3) were detected upstream or downstream of fosA7.5. The phylogenetic analysis revealed that both fosA7.5- and fosA3-carrying E. coli ST602 and fosA7.5-carrying E. coli ST2599 were closely related to E. coli isolates from humans, which may indicate that they pose a threat to human health.
IMPORTANCE Here, we report the widespread and complex genetic environments of fosA-like genes in animal-derived strains in China. The fosA7.5 gene was identified in this study and was found to confer resistance to fosfomycin. The high prevalence of fosA-like genes in farms indicates that food animals serve as a potential reservoir for the resistance genes. This study also discovered that fosfomycin resistance genes were always associated with mobile elements, which would accelerate the transmission of fosA-like genes in strains. Importantly, E. coli ST602 and ST2599 carrying fosA3 or fosA7.5 from food animals had high similarity to E. coli isolates from humans, suggesting that fosA-like genes can be transmitted to humans through the food chain, thus posing a serious threat to public health. Therefore, the prevalence of fosA-like genes isolated from animals should be further monitored.
KEYWORDS: food animals, Enterobacteriaceae, fosA-like genes, fosfomycin resistance, transmission, farms, fosfomycin, genetic environments
INTRODUCTION
The wide spread of multidrug-resistant (MDR) Gram-negative bacteria, such as extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae and carbapenem-resistant Enterobacteriaceae (CRE), has resulted in fewer options for clinical treatment. In this case, fosfomycin, an older antibiotic agent, has garnered renewed interest and is considered a first-line antibiotic to treat infections caused by carbapenem-resistant and polymyxin-resistant bacteria (1). However, as the use of fosfomycin increased, so did the widespread dissemination of fosfomycin-resistant isolates in some countries. It has already been reported that fosfomycin resistance is relatively severe in China, with the resistance rates ranging from 25% to 50% (2–4). However, fosfomycin is still effective against ESBL-producing Enterobacteriaceae such as Salmonella, Escherichia coli, and Klebsiella pneumoniae in Europe, the Americas, and Africa (5).
Resistance to fosfomycin is primarily mediated by the expression of fosfomycin-modifying enzymes (FosA, FosB, and FosC), whereas the FosA enzyme encoded by chromosomes or plasmids is the most common in Gram-negative bacteria. To date, more than 10 fosA-like genes (fosA1 to fosA10) have been identified, of which fosA3 encodes the primary mechanism leading to fosfomycin resistance of E. coli and K. pneumoniae in China (6–8). Presently, fosA3 is widely distributed among Enterobacteriaceae strains isolated from pets, pigs, chickens, and cows, as well as humans, although fosfomycin is not approved for use in animals in China (9–11). Furthermore, the coexistence of fosA3 with other antibiotic resistance determinants (blaCTX-M, blaTEM, and floR) on plasmids has resulted in the emergence of fosfomycin-resistant strains in various countries around the world (12).
Previous research discovered that fosA7 is mainly found on the chromosomes of Salmonella from various sources (human, cattle, sheep, and environment) (13). Subsequently, this gene was detected in different countries (14–16). In 2020, a study reported that the FosA identified in Escherichia coli differed from FosA7 protein, which was first reported in Salmonella, and its encoding gene was named fosA7.5Q86E (17). At present, fosA7.5 mainly exists in E. coli, and three variants of fosA7.5 were discovered, of which fosA7.5Q86E and fosA7.5WT demonstrated resistance to fosfomycin. Moreover, the fosfomycin resistance gene fosA described in Serratia marcescens in 1980, which was the first fosA-like gene (namely, fosA1), also could confer high-level resistance to fosfomycin (18). However, limited information is available regarding the prevalence of fosA and fosA7 among Enterobacteriaceae isolated from food animals, and no study has ever reported that fosA3 and fosA7.5 are coharbored in a single E. coli strain.
As a result, the strains containing fosA, fosA3, or fosA7.5 from food animals and their environments were analyzed in this study to better understand their resistance phenotypes, plasmid replicon typing, genetic environments, and transmission characteristics. It provides a scientific foundation for future efforts to prevent the spread of fosfomycin resistance genes at the human-animal-environment interface.
RESULTS
Identification of fosfomycin resistance determinants and coexisting resistance genes.
In this study, a total of 200 fosfomycin-resistant Enterobacteriaceae isolates were obtained from the samples. Among these 200 strains, 82 were positive for fosA-like genes, and they came from chicken feces (n = 36), pig feces (n = 6), sewage from pig farms (n = 3), pig lungs (n = 4), pig nose (n = 4), pig mouth (n = 6), soil from pig farms (n = 4), soil from chicken farms (n = 4), pig anus (n = 1), pigeon (n = 12), and shells of chicken eggs (n = 2). Among the 82 fosA-like-gene-positive isolates, including 52 fosA3-positive E. coli isolates (26%; 52/200), 10 fosA7.5-positive E. coli isolates from pigeons (10%; 10/200), and 20 fosA-positive isolates (Enterobacter cloacae (n = 10), Escherichia hormaechei (n = 7) and Escherichia asburiae (n = 3) isolates) were also identified by 16S rRNA sequencing. Importantly, in the 10 fosA7.5-harboring E. coli isolates, three strains (KPg84, fEc.1, and ECg85) coharbored both fosA7.5 and fosA3. However, fosC2 and other fosfomycin resistance genes were not detected. Detailed information for the 82 strains is shown in Table S1 in the supplemental material.
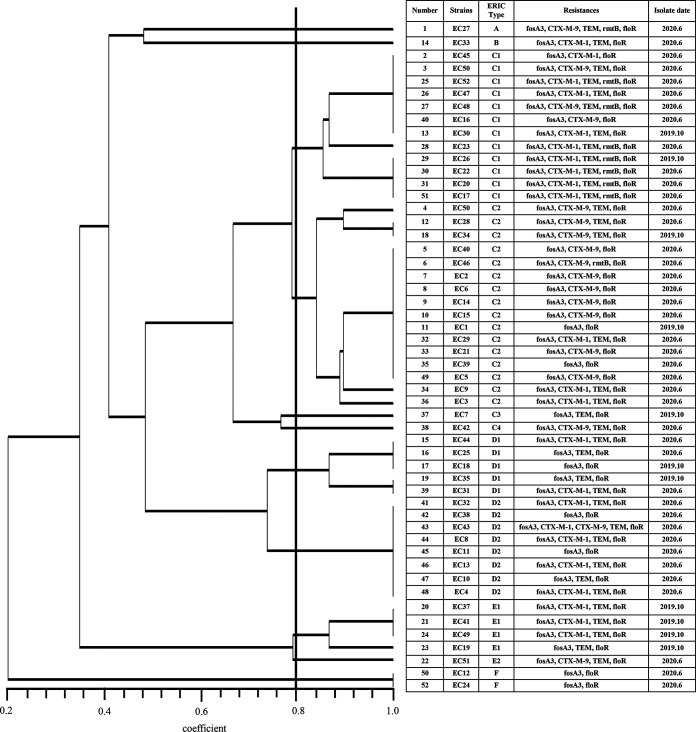
The fosA/fosA3/fosA7.5-carrying Enterobacteriaceae isolates were also tested for the presence of other significant antibiotic resistance genes (ARGs). Screening for resistance genes confirmed that 40 of the 52 fosA3-positive E. coli isolates carried blaCTX-like resistance genes, and strain EC43 contained two different blaCTX-M genes, including blaCTX-M-1G and blaCTX-M-9G. In addition, 9 and 35 isolates harbored rmtB and blaTEM genes, respectively, and all fosA3-carrying isolates were positive for floR. As a result, we identified the following gene combinations for fosA3: fosA3-blaCTX-M-1-blaTEM-rmtB-floR (n = 7), fosA3-blaCTX-M-9-blaTEM-rmtB-floR (n = 2) fosA3-blaCTX-M-9-blaTEM-floR (n = 5), fosA3-blaCTX-M-1-blaTEM-floR (n = 15), fosA3-blaCTX-M-1-blaCTX-M-9-blaTEM-floR (n = 1), fosA3-floR (n = 6), fosA3-blaTEM-floR (n = 6), fosA3-blaCTX-M-1-floR (n = 1), fosA3-blaCTX-M-9-floR (n = 8), and fosA3-blaCTX-M-9-rmtB-floR (n = 1) (Table 1; also, see Fig. 1). Except for strains ECg29 and EC315, all other fosA7.5-positive E. coli isolates carried blaCTX-M, blaTEM, and floR genes, and the most frequent gene profile was fosA3/fosA7.5-blaCTX-M-1/CTX-M-9-floR-blaTEM (n = 8) (Table 2). However, most of the 20 fosA-positive strains showed a single-gene profile, and only one and four strains carried blaNDM- and blaCTX-like resistance genes, respectively (Table 2). The rates of floR, blaTEM, and rmtB genes were relatively low, at 20% (4/20), 10% (2/20), and 5% (1/20).
TABLE 1.
Characterization of 29 conjugable fosA3-positive E. coli isolates
| Strains | Context of fosA3a | Resistance profileb | Resistance genes |
|---|---|---|---|
| EC27 | V | FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-9, blaTEM, rmtB, floR |
| EC28 | V | FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-9, blaTEM, floR |
| EC29 | I | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC30 | I | CAZ, FFC, CHL, TET, TGC, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC31 | II | CAZ, FFC, CHL, TET, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC32 | II | CAZ, FFC, CHL, TET, CIP, AMK, COL, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC33 | IV | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC34 | V | FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-9, blaTEM, floR |
| EC35 | VI | FFC, CHL, TET, CIP, FOS | fosA3, blaTEM, floR |
| EC36 | VI | CAZ, FFC, CHL, TET, CIP, AMK, FOS | fosA3, blaCTX-M-1, blaTEM, rmtB, floR |
| EC37 | I | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC38 | VI | FFC, TET, FOS | fosA3, floR |
| EC39 | II | FFC, CHL, TET, CIP, TGC, FOS | fosA3, blaTEM, floR |
| EC40 | IV | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-9, floR |
| EC41 | VI | CAZ, FFC, CHL, TET, CIP, TGC, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC42 | V | FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-9, blaTEM, floR |
| EC43 | IV | CAZ, FFC, CHL, TET, CIP, COL, FOS | fosA3, blaCTX-M-1, blaCTX-M-9, blaTEM, floR |
| EC44 | II | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC45 | I | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-1, floR |
| EC46 | VI | CAZ, FFC, CHL, TET, CIP, TGC, FOS | fosA3, blaCTX-M-9, rmtB, floR |
| EC47 | II | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC48 | II | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-9, blaTEM, rmtB, floR |
| EC49 | VI | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-1, blaTEM, floR |
| EC50 | V | FFC, CHL, TET, CIP, FOS | fosA3, blaCTX-M-9, blaTEM, floR |
| EC51 | VI | FFC, CHL, TET, CIP, TGC FOS | fosA3, blaCTX-M-9, blaTEM, floR |
| EC52 | I | FFC, CHL, TET, AMK, TGC, FOS | fosA3, blaCTX-M-1, blaTEM, rmtB, floR |
| Kpg84 | / | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, fosA7.5, blaCTX-M-1, floR, blaTEM |
| fEc.1 | III | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, fosA7.5, blaCTX-M-1, floR, blaTEM |
| ECg85 | / | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, fosA7.5, blaCTX-M-1, floR, blaTEM |
/, the genetic environment of fosA3 was not detected.
CAZ, ceftazidime; FFC, florfenicol; CHL, chloramphenicol; TET, tetracycline; CIP, ciprofloxacin; AMK, amikacin; COL, colistin; TGC, tigecycline; MEM, meropenem; FOS, fosfomycin.
FIG 1.
ERIC-PCR profiles of 52 fosA3-positive E. coli isolates.
TABLE 2.
Characterization of 10 fosA7.5-positive isolates and 20 fosA-positive isolates
| Strain | Resistance profilea | Resistance gene(s) |
|---|---|---|
| Kpg84 | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, fosA7.5, blaCTX-M-1, floR, blaTEM |
| fEc.1 | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, fosA7.5, blaCTX-M-1, floR, blaTEM |
| ECg85 | CAZ, FFC, CHL, TET, CIP, FOS | fosA3, fosA7.5, blaCTX-M-1, floR, blaTEM |
| fEcg991 | CAZ, FFC, CHL, TET, CIP, FOS | fosA7.5, blaCTX-M-9, floR, blaTEM |
| ECg29 | CAZ, FFC, CHL, TET, CIP, FOS | fosA7.5, floR, blaTEM |
| ECg931 | CAZ, FFC, CHL, TET, CIP, FOS | fosA7.5, blaCTX-M-1, floR, blaTEM |
| ECg932 | CAZ, FFC, CHL, TET, CIP, FOS | fosA7.5, blaCTX-M-1, blaCTX-M-9, floR |
| ECg91 | CAZ, FFC, CHL, TET, CIP, AMK, FOS | fosA7.5, blaCTX-M-1, blaCTX-M-9, floR |
| ECg933 | CAZ, FFC, CHL, TET, CIP, FOS | fosA7.5, blaCTX-M-1, blaCTX-M-9, floR |
| EC315 | FFC, FOS | fosA7.5, floR, blaTEM |
| 20E.1 | FFC, FOS, TET | fosA |
| 20E.2 | FFC, TGC, FOS, TET | fosA |
| EC2088 | FFC, CHL, TET, TGC, FOS | fosA, floR |
| 20E.4 | FFC, CHL, TET, TGC, FOS | fosA, floR |
| 20E.5 | FFC, TET, TGC, FOS | fosA, blaTEM |
| 20E.6 | FFC, COL, TGC, FOS | fosA |
| 20E.7 | TET, COL, TGC, FOS | fosA |
| 20E.8 | TET, COL, TGC, FOS | fosA |
| 20E.9 | FFC, TET, COL, TGC, FOS | fosA, blaCTX-M-9 |
| EC2098 | FFC, CHL, TET, CIP, FOS | fosA, floR |
| 20E.11 | FFC, CHL, TET, FOS | fosA |
| KP20117 | FFC, CHL, TET, COL, TGC, FOS | fosA |
| 20E.13 | CAZ, FFC, CHL, TET, CIP, COL, TGC, FOS | fosA, blaCTX-M-9 |
| 20E.14 | CAZ, FFC, CHL, TET, CIP, COL, TGC, FOS | fosA, blaCTX-M-9 |
| 20E.15 | CAZ, FFC, CHL, TET, CIP, AMK, FOS | fosA, rmtB |
| 20E.16 | CAZ, TET, CIP, COL, FOS | fosA |
| 20E.17 | CAZ, FFC, CHL, TET, COL, TGC, FOS | fosA, blaTEM, floR, blaCTX-M-1 |
| 20E.18 | CAZ, TET, MEM, FOS | fosA, blaNDM |
| EC1928 | FFC, CHL, TET, CIP, TGC, FOS | fosA |
| 20E.20 | FFC, COL, TGC, FOS | fosA |
CAZ, ceftazidime; FFC, florfenicol; CHL, chloramphenicol; TET, tetracycline; CIP, ciprofloxacin; AMK, amikacin; COL, colistin; TGC, tigecycline; MEM, meropenem; FOS, fosfomycin.
Detection of antimicrobial resistance patterns.
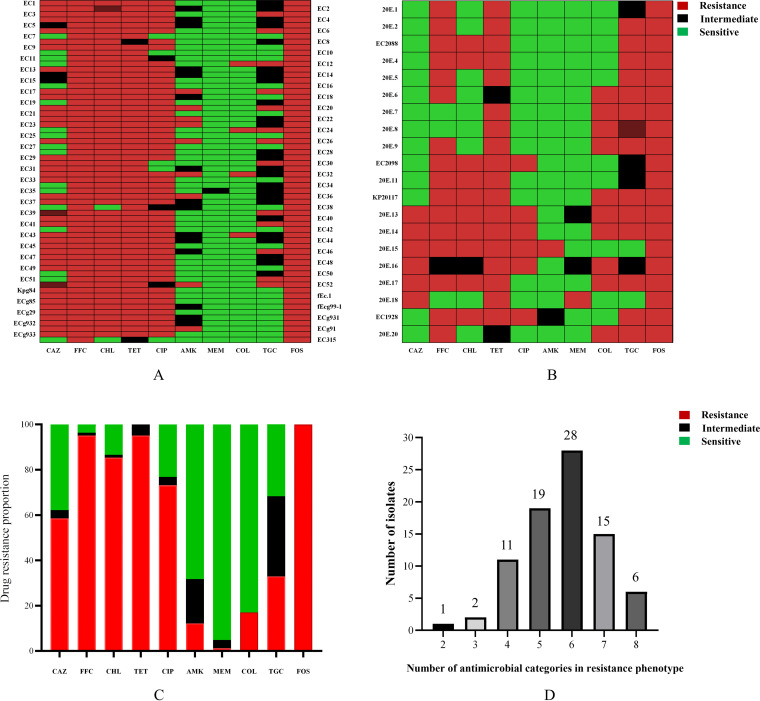
In this study, 82 Enterobacteriaceae isolates containing fosA-like genes showed different degrees of resistance to 10 antimicrobial agents (Fig. 2A and B). Susceptibility testing indicated that all 82 strains were resistant to fosfomycin (100%; 82/82). These fosfomycin-resistant isolates also showed resistance to other antibiotics, such as ceftazidime (58.54%; 48/82), florfenicol (95.12%; 78/82), and chloramphenicol (85.37%; 70/82), tetracycline (90.24%; 74/82) and ciprofloxacin (73.17%; 60/82), and the resistance rates were all above 55%. However, only one and 10 strains were resistant to meropenem (1.22%) and amikacin (12.20%), respectively. It was also found that 16 strains (19.51%) exhibited intermediate resistance to amikacin (MIC = 4 μg/mL). Furthermore, several isolates were resistant to colistin (17.07%; 4/62) and tigecycline (20.97%; 14/82), with MICs at or above 2 μg/mL, and the resistant strains were mostly detected among fosA-positive isolates (Fig. 2C). Except for one strain that was only resistant to two antibiotics (fosfomycin and florfenicol), all 81 strains carrying fosA-like genes were multidrug-resistant strains (resistant to at least 3 classes of agents). According to the findings, 79 strains (98.75%) were resistant to 4 or more antibiotics, and six strains were resistant to all 8 antibiotics (Fig. 2D). The MICs of 82 strains are listed in Table S2 and S3.
FIG 2.
Analysis of the susceptibility results of 82 Enterobacteriaceae isolates with fosfomycin resistance for 13 antibiotics. (A and B) Drug resistance spectrum; (C) drug resistance proportion; (D) numbers of isolates with given numbers of antimicrobial categories in the resistance phenotypes.
Conjugation experiments and plasmid analysis.
Among the 82 fosA/fosA3/fosA7.5-harboring isolates, 29 (35.37%; 29/82) were able to successfully transfer the fosfomycin resistance phenotype to E. coli recipient strain C600, and all transconjugants carried fosA3. For the three E. coli isolates coharboring both fosA7.5 and fosA, only fosA3 was successfully transferred from three donors to the recipient. Moreover, no fosA or fosA7.5 transconjugants were acquired, indicating that these genes may be located on chromosomes or nonconjugative plasmids of strains. The MICs of 7 antimicrobial agents for 29 transconjugants are listed in Table 3, all of which were resistant to fosfomycin (MIC > 512 μg/mL). Furthermore, the 29 transconjugants showed resistance to ceftazidime (62.07%; 18/29), florfenicol (86.21%; 25/29), chloramphenicol (86.21%; 25/29), tetracycline (68.97%; 20/29), ciprofloxacin (17.24%; 5/29), and amikacin (10.34%; 3/29). It was found that 24 (82.76%) fosA3-carrying transconjugants were resistant to more than 4 antibiotics (Fig. S3). Enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) indicated that the bands of conjugants were consistent with E. coli C600, while showing differences with the donors (Fig. S4).
TABLE 3.
MICs of 10 antimicrobial agents for the 29 fosA3 transconjugants
| Strain | MIC (μg/mL) ofa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| CAZ | FFC | CHL | TET | CIP | AMK | RIF | FOS | |
| EC27-T | <1 | 128 | 32 | 2 | <1 | <1 | >1,000 | >512 |
| EC28-T | <1 | 256 | 128 | 64 | <1 | 2 | >1,000 | >512 |
| EC29-T | 8 | 2 | 2 | 2 | <1 | <1 | >1,000 | >512 |
| EC30-T | 16 | 512 | 128 | 128 | <1 | 2 | >1,000 | >512 |
| EC31-T | 4 | 256 | 64 | 32 | <1 | <1 | >1,000 | >512 |
| EC32-T | 16 | 4 | 2 | 2 | <1 | 2 | >1,000 | >512 |
| EC33-T | 8 | 256 | 256 | 32 | <1 | <1 | 125 | >512 |
| EC34-T | <1 | 128 | 64 | 64 | <1 | <1 | >1,000 | >512 |
| EC35-T | <1 | 256 | 128 | 128 | 8 | 2 | >1,000 | >512 |
| EC36-T | 8 | 256 | 64 | 64 | <1 | <1 | >1,000 | >512 |
| EC37-T | 16 | 512 | 128 | 256 | <1 | <1 | >1,000 | >512 |
| EC38-T | <1 | 512 | 256 | 256 | <1 | <1 | >1,000 | >512 |
| EC39-T | 8 | 512 | 256 | 128 | 512 | <1 | 1,000 | >512 |
| EC40-T | 16 | 512 | 256 | 256 | 128 | 2 | >1,000 | >512 |
| EC41-T | 8 | 256 | 64 | 128 | <1 | 2 | >1,000 | >512 |
| EC42-T | <1 | 128 | 64 | 32 | <1 | 2 | >1,000 | >512 |
| EC43-T | 16 | 2 | 2 | 2 | <1 | <1 | >1,000 | >512 |
| EC44-T | 8 | 4 | 2 | 64 | <1 | <1 | >1,000 | >512 |
| EC45-T | 8 | 256 | 128 | 16 | <1 | <1 | >1,000 | >512 |
| EC46-T | 8 | 256 | 64 | 512 | 64 | 2 | >1,000 | >512 |
| EC47-T | <1 | 8 | 64 | 4 | <1 | 8 | >1,000 | >512 |
| EC48-T | <1 | 8 | 64 | 4 | <1 | 16 | >1,000 | >512 |
| EC49-T | 16 | 512 | 256 | 256 | <1 | <1 | >1,000 | >512 |
| EC50-T | <1 | 128 | 64 | 64 | <1 | <1 | >1,000 | >512 |
| EC51-T | <1 | 256 | 128 | 64 | <1 | <1 | >1,000 | >512 |
| EC52-T | 8 | 128 | 128 | 64 | 2 | 8 | >1,000 | >512 |
| fEc.1-T | 8 | 256 | 128 | 2 | <1 | <1 | >1,000 | >512 |
| Kpg84-T | 8 | 256 | 64 | <1 | <1 | <1 | >1,000 | >512 |
| ECg85-T | 8 | 256 | 128 | 2 | <1 | <1 | >1,000 | >512 |
| C600 | <1 | <1 | 2 | 2 | <1 | <1 | >1,000 | <1 |
CAZ, ceftazidime; FFC, florfenicol; CHL, chloramphenicol; TET, tetracycline; CIP, ciprofloxacin; AMK, amikacin; RIF, rifampicin; FOS, fosfomycin.
Except for three strains that carried both fosA3 and fosA7.5, 26 conjugable fosA3-positive E. coli isolates included a total of 8 different plasmid replicon types, including Inc (I1, FIA, FIB, FII, K, HI1, HI2, N), and all of the strains contained 2 or more plasmid replicons (Table 4). The corresponding transconjugants also acquired different plasmid replicons; only IncFIB replicons were detected in two transconjugants (EC47-T and EC48-T), indicating that fosA3 was located on Inc(FIB) plasmids. The results showed that multiple plasmids were transferred horizontally with the fosA3 plasmids. Different from fosA3-positive isolates, seven plasmid replicons were detected in 10 fosA7.5-positive E. coli isolates (Table S4), including Inc (FrepB, FIB, FII, I1, K, and Y). FrepB was discovered in all fosA7.5-positive E. coli strains, while IncY was found in six strains.
TABLE 4.
Plasmid replicons of the 29 fosA3-positive E. coli and their transconjugants
| Strain | Plasmid types | Transconjugant | Plasmid type(s) |
|---|---|---|---|
| EC27 | HI2, FIB, FII, K | EC27-T | FIB, FII |
| EC28 | FIB, FII, K | EC28-T | FIB, FII |
| EC29 | I1, FIA, FIB, FII, K | EC29-T | I1, FIB, FII |
| EC30 | I1, FIB, FII, K | EC30-T | FIB, FII |
| EC31 | FIB, FII, K | EC31-T | FIB, FII |
| EC32 | HI1, HI2, N, FIB, FII | EC32-T | N, FIB, FII |
| EC33 | HI1, FIB, FII, K | EC33-T | FIB, FII |
| EC34 | FIB, FII, K | EC34-T | FIB, FII |
| EC35 | FIB, FII | EC35-T | FIB, FII |
| EC36 | N, FIB, B, FII, K | EC36-T | N, FIB, FII |
| EC37 | FIB, FII, K | EC37-T | FIB, FII |
| EC38 | HI1, FIB, FII | EC38-T | FIB, FII |
| EC39 | FIB, FII, K | EC39-T | FIB, FII |
| EC40 | FIB, FII, K | EC40-T | FIB, FII |
| EC41 | FIB, FII, K | EC41-T | FIB, FII |
| EC42 | HI2, FIB, FII, K | EC42-T | FIB, FII |
| EC43 | HI1, HI2, N, FIB, FII | EC43-T | N, FIB, FII |
| EC44 | FIB, FII, K | EC44-T | FIB, FII |
| EC45 | HI1, FIB, FII, K | EC45-T | FIB, FII |
| EC46 | FIB, FII, K | EC46-T | FIB, FII |
| EC47 | I1, N, FIB, B, FII, K | EC47-T | FIB |
| EC48 | I1, N, FIB, B, FII, K | EC48-T | FIB |
| EC49 | FIB, FII, K | EC49-T | FIB, FII |
| EC50 | FIB, FII, K | EC50-T | FIB, FII |
| EC51 | FIB, FII, K | EC51-T | FIB, FII |
| EC52 | I1, FIB, FII, K | EC52-T | FIB, FII |
| Kpg84 | FrepB, FIB, FII, I1, K | Kpg84-T | I1, FIB, FII |
| fEc.1 | FrepB, FIB, FII, I1, K | Ecg87-T | I1, FIB, FII |
| ECg85 | FrepB, FIB, I1, FII, K | Kpg85-T | I1, FIB, FII |
| fEcg99-1 | FrepB, FIB, I1, Y, FII, K | None | None |
Strain typing (ERIC-PCR and MLST).
The genomic diversity analysis of 52 fosA3-positive strains and 10 fosA7.5-positive strains was analyzed by using the ERIC-PCR fingerprinting method. Among the 52 fosA3-positive E. coli isolates, the number of amplified bands ranged from 3 to 10, with sizes of 100 bp to 2,000 bp, and the genetic similarity was 20% to 100%. These isolates were divided into 6 main clusters (A to F) and 11 ERIC types, of which cluster C (C1 to C4) was the dominant genotype (59.62%; 31/52), and most of the strains in cluster C were derived from animal feces. Clusters A and B had the fewest strains, with only one strain in each cluster. The remaining five clusters (D to F) contained 13 (25%), 5 (9.62%) and 1 (3.85%) isolates, respectively (Fig. 1). MLST revealed a new sequence type (ST) and 15 known STs for the 29 conjugable fosA3-positive E. coli isolates, in which ST115 was the most common (n = 5), followed by ST156 (n = 4), ST7069 (n = 3), ST117 (n = 3), ST1196 (n = 2), ST23 (n = 2). Other STs were ST5229, ST683, ST202, ST224, ST410, ST1148, ST602, ST1468, and ST48, and each ST had one isolate. The two known STs (ST410 and ST23) belong to clonal complex 23 (CC23) and had only one difference in their purA alleles. The allele profiles of STs are provided in Table 5.
TABLE 5.
The ST types and of conjugable fosA3-positive E. coli and fosA7.5-carrying isolates
| No. of allele genes |
STa | Strain(s) | ||||||
|---|---|---|---|---|---|---|---|---|
| adk | fumC | gyrB | icd | mdh | purA | recA | ||
| 6 | 6 | 33 | 26 | 11 | 8 | 2 | 1196 | EC27, EC42 |
| 6 | 4 | 14 | 16 | 24 | 8 | 14 | 115 | EC28, EC34, EC35, EC44, EC50 |
| 112 | 11 | 5 | 12 | 8 | 8 | 6 | 7069 | EC37, EC41, EC49 |
| 6 | 11 | 4 | 8 | 8 | 8 | 2 | 48 | EC52 |
| 43 | 41 | 15 | 18 | 11 | 7 | 44 | 5229 | EC29 |
| 20 | 45 | 41 | 43 | 5 | 32 | 2 | 117 | EC32, EC38, EC43 |
| 6 | 4 | 12 | 1 | 20 | 13 | 7 | 23 | EC47, EC48 |
| 6 | 4 | 127 | 16 | 24 | 8 | 6 | 683 | EC30 |
| 6 | 4 | 12 | 1 | 20 | 18 | 7 | 410 | EC36 |
| 6 | 4 | 33 | 16 | 11 | 8 | 6 | 224 | EC33 |
| 6 | 95 | 3 | 18 | 11 | 7 | 14 | 1148 | EC45 |
| 6 | 29 | 32 | 16 | 11 | 8 | 44 | 156 | EC40, EC46, EC39, EC51 |
| 64 | 11 | 5 | 8 | 5 | 8 | 2 | 202 | EC31 |
| 6 | 19 | 33 | 26 | 11 | 8 | 6 | 602 | fEC.1 |
| 6 | 6 | 153 | 26 | 11 | 8 | 6 | 1468 | ECg85 |
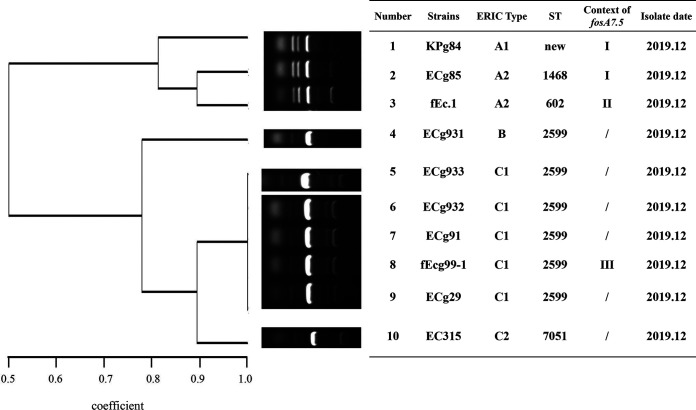
| 267 | 6 | 5 | 26 | 9 | 13 | 98 | 2599 | fEcg99-1, ECg931, ECg933, ECg932, ECg91, ECg29 |
| 6 | 19 | 33 | 26 | 11 | 8 | 98 | NA | Kpg84 |
| 653 | 19 | 270 | 26 | 11 | 8 | 7 | 7051 | EC315 |
NA, no ST type of the strain has been obtained.
MLST analysis showed that the 10 fosA7.5-positive E. coli isolates belonged to 4 STs (one ST1468, one ST602, six ST2599, and one ST7051), in which ST2599 was predominant. Analysis of the ERIC-PCR profiles showed that there were a total of 3 unique clusters (A, B, and C) and 5 ERIC types within 10 fosA7.5-carrying E. coli isolates. Except for strain ECg931, other ST2599 and ST7051 strains belonged to cluster C. Also, the three E. coli isolates carrying both fosA3 and fosA7.5 were classified as cluster A (Fig. 3). ERIC-PCR combined with MLST analyses indicated that the fosA-like genes were spread by horizontal transfer as well as via clonal transmission between Enterobacteriaceae isolates in the farms. The allele profiles of STs are provided in Table 5.
FIG 3.
ERIC-PCR profiles of 10 fosA7.5-positive E. coli isolates.
Genetic background of fosA in E. cloacae and E. hormaechei.
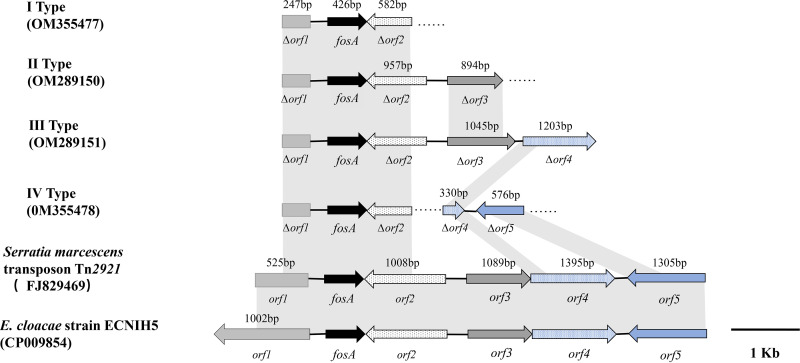
For the 17 fosA-positive isolates, four types of genetic contexts were identified by PCR mapping, all of which shared > 99% similarity with partial sequences in E. cloacae strain ECNIH5 (CP009854) and S. marcescens transposon Tn2921 (FJ829469). The most common were type I (n = 7) and type III (n = 3), while the others were type II (n = 1) and type IV (n = 1). In all four types, a 247-bp length of amplicon in the upstream region of fosA was identical to the truncated tryptophan tRNA synthetase gene in E. cloacae strain ECNIH5 and transposon Tn2921. In the downstream region of fosA, we found four amplicons with lengths of 957 bp, 894/1,045 bp, 1,203 bp, and 576 bp encoding the LacI family transcriptional regulator, sugar-binding cellulose-like protein, MFS sugar transporter, and restriction endonuclease, respectively, which were similar to part of the sequence in transposon Tn2921. In type I, only a 582-bp length of amplicon encoding the LacI family transcriptional regulator was confirmed in the downstream region of fosA (Fig. 4).
FIG 4.
Genetic contexts of fosA in E. cloacae and E. hormaechei. orf1, orf2, orf3, orf4, and orf5 encode part of the tryptophan tRNA synthetase, LacI family transcriptional regulator, glycosyl hydrolase family 2, MFS sugar transporter, and a restriction endonuclease. Shaded boxes between sequences indicate homologous regions (>90% sequence identity).
Genetic background of fosA3 in E. coli isolates.
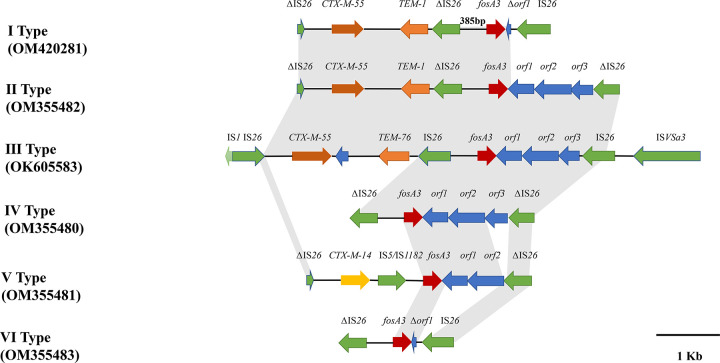
PCR mapping was used to determine the regions adjacent to fosA3 in 26 conjugable fosA3-positive E. coli isolates. Five different genetic contexts were identified, including type I (n = 4), type II (n = 6), type IV (n = 3), type V (n = 6), and type VI (n = 7) (Fig. 5). The fosA3 gene was flanked by two IS26 elements oriented in the opposite direction in 20 isolates. An IS26 element was found to be located on downstream of fosA3 in all isolates, and the lengths of the spacer regions between the 3′ end of fosA3 and the IS26 gene were variable (2,377 bp, 1,823 bp, and 707 bp). In type I, II, IV, and VI structures, the IS26 element was located 385 bp upstream of fosA3. In addition, the extended-spectrum β-lactamase (ESBL) gene blaCTX-M-55 was frequently located upstream of fosA3 in two genomic contexts (type I and type II), and a truncated IS26 transposase determinant was identified upstream of blaCTX-M-55. The type V (n = 6) structure was from blaCTX-M-14-positive isolates; it was found that the IS26 element upstream of fosA3 was replaced by the ΔIS26-blaCTX-M-14-IS5/IS1182-fosA3 structure, which was similar to that on plasmid on LWY24 (MT318677.1, chicken, E. coli) (Fig. 5).
FIG 5.
Genetic context of fosA3 in E. coli. Arrows indicate the directions of transcription of the genes, and different genes are shown in different colors. Shaded boxes between sequences indicate homologous regions (>90% sequence identity). orf1, orf2, and orf3 encode a hypothetical protein, a CadC-like protein, and a truncated TetR family transcriptional regulator.
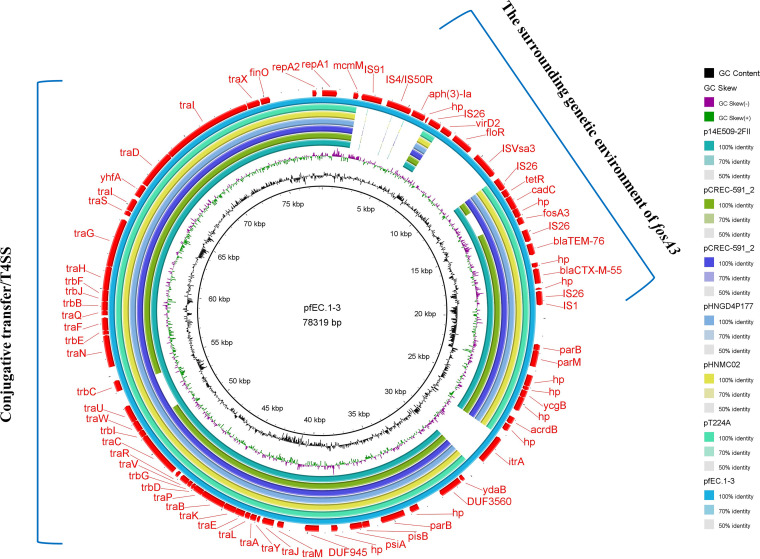
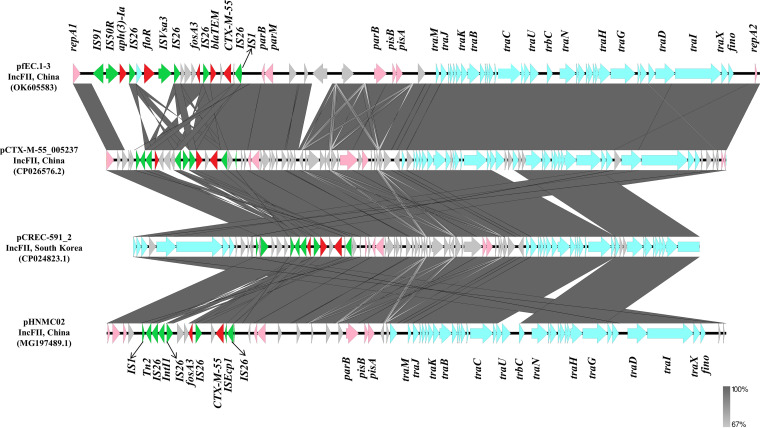
One representative pigeon-derived E. coli isolate (fEC.1) carrying fosA3 and fosA7.5 was analyzed by whole-genome sequencing (WGS) and was identified as ST602. The fosA3-harboring plasmid was named pfEC.1-3 (OK605583), with a size of 78,319 bp. The plasmid belonged to the IncFII incompatibility group and contained a variable region responsible for fosA3. The two structures, IS1-IS26-orf-blaCTX-M-55-orf-blaTEM-76-IS26-fosA3 and fosA3-orf1-orf2-orf3-IS26-ISVsa3/IS91-floR-aph(3′)-Ia, were located upstream and downstream of fosA3, respectively, and were named type III. A BLAST search for pfEC.1-3 revealed highly homology (>90%) to six other known IncFII plasmids deposited in the GenBank database, which were p14E509-2FII (MN822125.1; China; human), pCREC-591_2 (CP024823.1; South Korea; human), pCTX-M-55_005237 (CP026576.2; China; human), pHNGD4P177 (MG197492.1; China; pig), pHNMC02 (MG197489.1; China; chicken) and pT224A (MW298658.1; Canada; dairy cow). All plasmids had backbone genes associated with IncFII plasmid replication (repA1/A2), conjugative transfer and the type IV secretion system (T4SS) (tra and trb), separation (parM), and maintenance of genetic stability (stbA) (Fig. 6). However, the variations between these plasmids resulted from insertion sequences (IS26, IS4, and IS91), integrase (IntI), and resistance [floR and aph(3′)-Ia] genes around the fosA3 gene (Fig. 7).
FIG 6.
Comparative genomics analysis of IncFII plasmids carrying fosA3, the external ring represents the annotation of pfEC.1-3.
FIG 7.
Comparison of the genetic environment of fosA3 in pfEc.1-3 and other closely related IncFII plasmids.
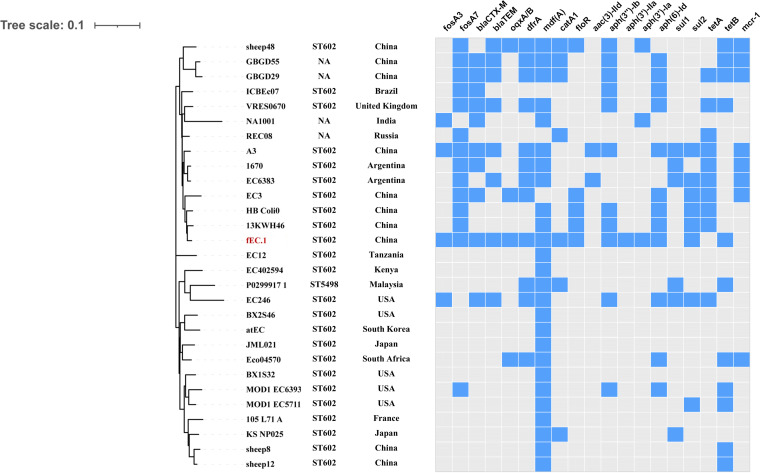
Phylogenetic analysis of fEC.1.
Phylogenetic analysis was performed by using WGS information available in the GenBank database for ST602 fEC.1 and 28 E. coli isolates (ST602, n = 23; ST5498, n = 1; ST unknown, n = 4) from clinical samples from different sources, including humans, animals, and plants, and one E. coli isolate of unknown origin. The phylogenetic analysis by core genome MLST (cgMLST) revealed that ST602, ST5498 and 4 unknown STs were classified into the same lineage, indicating clonal spread of these strains. Isolate fEC.1 from this study was most closely related to two ST602 E. coli isolates, 13KWH46 (CP019250) and HB_Coli0 (CP020933), collected from a patient and chicken feces, which both carried fosA7, mdf(A), floR, aph(3′)-IIb, aph(6′)-Id, sul2, tet(A), and tet(B) genes. Importantly, these strains were also abundant in distribution, including some countries in Asia, Africa, North America, and Europe, suggesting that an ST602 E. coli isolate is spreading across host species and continents. In addition, the majority of the strains carried fosfomycin resistance genes, including fosA3 and fosA7, and showed a multiresistance gene profile (Fig. 8).
FIG 8.
Phylogenetic relationship of ST602 E. coli isolate fEC.1 (in red) from this study with ST602 isolates from China and other countries. Blue and gray squares indicate the presence and absence of antimicrobial resistance genes, respectively.
Genetic background of fosA7.5 in E. coli isolates.
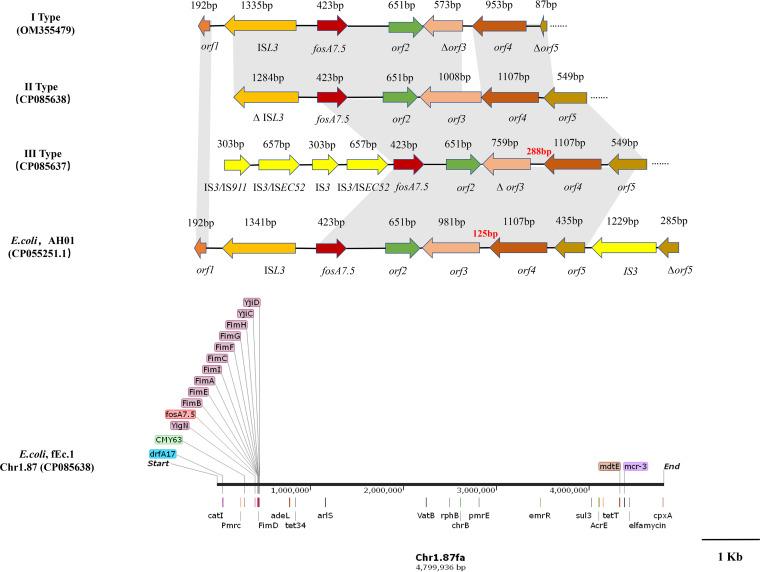
In addition to strain fEC.1, a fosA7.5-carrying E. coli isolate (fEcg99-1) from a pigeon was also completely sequenced to analyze the genetic environment of fosA7.5. The MLST scheme revealed that fEcg99-1 belonged to sequence type ST2599. This study identified 3 different genetic contexts associated with the fosA7.5 gene, designated type I to III (Fig. 9). WGS revealed that fosA7.5 was located on the chromosome in strains fEc.1 (type II) and fEcg99-1 (type III). In all three types, a gene sequence containing orf2, orf3, orf4, and orf5 was found downstream of fosA7.5 that encoded HNH endonuclease, sialate-O-acetylesterase, sialic acid-induced transmembrane protein YjhT (NanM), and N-acetylneuraminic acid outer membrane channel protein (NanC), respectively. According to comparative genomic analysis, the three structures (type I to III) were highly similar to part of E. coli AH01 (CP055251.1). However, the difference was that the orf3 sequence lengths in the three types were 573 bp, 1,008 bp, and 759 bp, respectively. The ISL3 element was found in the upstream region of fosA7.5 from isolates Kpg84, ECg85 (type I), and fEc.1 (type II), with lengths of 1,335 bp and 1,284 bp. Unlike the other two types, a sequence containing four IS3-like elements (IS911, 303 bp; ISEC52, 657 bp; IS911, 303 bp; and ISEC52, 657 bp) was confirmed upstream of fosA7.5 in type III. Also, the mobile elements of type III were highly similar to the IS3 element, located downstream of fosA7.5 in E. coli AH01, but the genetic direction is opposite (Fig. 9).
FIG 9.
Genetic context of fosA7.5 in E. coli. Arrows indicate the directions of transcription of the genes, and different genes are shown in different colors. Shaded boxes between sequences indicate homologous regions (>90% sequence identity). The letter Δ indicates a truncated gene.
The fosA7.5 gene from E. coli in this study was 100% identical to fosA7.5WT (wild-type fosA7.5 sequence; WP_000941933.1), whereas it differed from the novel fosA7 variant fosA7.5Q86E (EC623772). The antimicrobial susceptibility testing also confirmed that the 10 fosA7.5-positive E. coli isolates in this study showed high-level resistance to fosfomycin (MIC ≥ 512 μg/mL). In addition, fimbriae proteins (FimB, FimA, and FimE), bacterial membrane proteins (YijC and YijN), along with T3SS and T6SS secretion systems were identified on fosA7.5-bearing chromosomes in strains fEC.1 and fECg99-1, all of which were connected to bacterial virulence. Moreover, genes for the two-component regulatory systems, resistance, and efflux pumps related to antibiotic resistance were also found on the chromosomes, for example, genes for the two-component regulatory system Arls, PmcR, and PmrE and the mcr-3 gene involved in colistin resistance, as well as the ARGs blaCMY-63, sul3, tetA, and dfrA (Fig. 9).
Phylogenetic analysis of fEC.99-1.
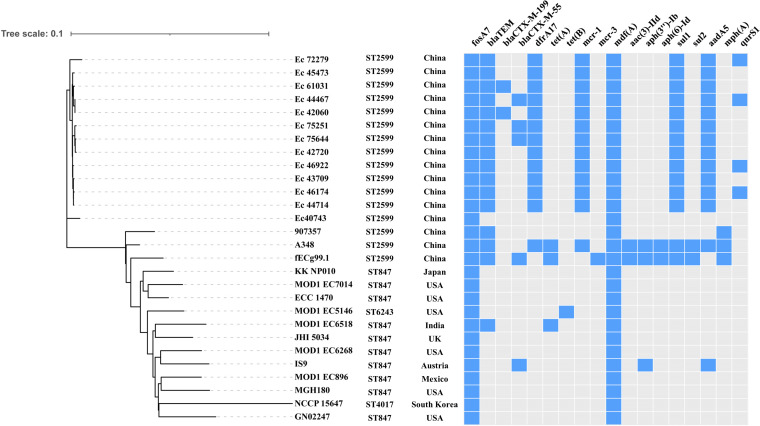
Similarly, the fosA7.5-carrying E. coli ST2599 strain fECg99-1 was studied by core genome MLST (cgMLST)-based phylogenomic analysis with 27 E. coli strains in GenBank (ST2599, n = 15; ST847, n = 10; ST6243, n = 1; ST4017, n = 1). ST2599 and ST847 have 6 identical alleles and differ only in their adk alleles. The results showed that the E. coli isolates from different parts of the world and multiple sources (human, cow, chicken, mouse, and pigeon) clustered together. Isolate fECg99-1 was found to be in the same lineage as ST2599 isolates from humans, in which two isolates 907357 (AXUH01) and A348 (NSAT01) collected from China were most similar to fECg99-1, indicating that the E. coli ST2599 strain has spread between humans and animals. Moreover, ST847 E. coli from Australia, the United States, India, and Mexico shared clonal similarities with fEC.99-1. In addition, all strains carried fosA7 and also showed a multiresistance gene profile (Fig. 10).
FIG 10.
Phylogenetic relationship of ST2599 E. coli isolate fECg99-1 from this study with isolates from China and other countries. Blue and gray squares indicate the presence and absence of antimicrobial resistance genes, respectively.
Finally, to determine whether fosA7.5 in this study could confer resistance to fosfomycin, we created a recombinant plasmid, pET-28a+fosA7.5 (Fig. S5). The fosfomycin MIC for E. coli Top10 transformed with pET-28a+fosA7.5 was >128 μg/mL, which was more than 64-fold higher than that for E. coli Top10 transformed with pET-28a alone (Table 6).
TABLE 6.
MICs for constructed and original strains
| Antibiotic | MIC (μg/mL) for fEC.1 |
||
|---|---|---|---|
| Alone | With pET-28a(+)-fosA7.5-Top10 | With pET-28a(+)-Top10 | |
| Ceftazidime | 32 | <1 | <1 |
| Florfenicol | 512 | 4 | 2 |
| Chloramphenicol | 256 | 2 | 2 |
| Tetracycline | 32 | 8 | 4 |
| Ciprofloxacin | 128 | <1 | <1 |
| Amikacin | <1 | <1 | <1 |
| Colistin | <1 | <1 | <1 |
| Tigecycline | <0.25 | <0.25 | <0.25 |
| Meropenem | <1 | <1 | <1 |
| Ampicillin | >512 | 256 | 32 |
| Fosfomycin | >512 | >128 | 2 |
DISCUSSION
Fosfomycin has been used all over the world to treat clinical urinary tract infections. However, with the irregular use of antibiotics, the problem of fosfomycin resistance has gradually become serious. The use of fosfomycin in veterinary medicine has not been approved in China. However, this study revealed that the fosA-like genes in animal-derived Enterobacteriaceae isolates have a general prevalence, with fosA3 (26%) having the highest detection rate. This rate was higher than the previously reported positivity rate for fosA3 in humans, ducks, and pets (7, 10, 19). In addition, all strains containing fosA-like genes in this study exhibited a high-level resistance to fosfomycin (MIC ≥ 512 μg/mL).
According to previous reports (20, 21), fosA was frequently discovered on the chromosome of E. cloacae or on the transposon Tn2921 of S. marcescens, while data on fosA prevalence are lacking. In this study, a total of 20 fosA-positive strains were identified, with a rate of 10%. A recent study reported the discovery of fosA in pet-derived E. cloacae from Taiwan, China, and similar to this study, 2 strains carried both fosA and fosA3 (18). In addition, 10 fosA7.5-positive E. coli isolates were obtained from pigeons, and three of them also harbored fosA3. Since the identification of fosA7 on the chromosomes of Salmonella enterica serovar Heidelberg from chickens in 2017, it has been detected in different sources, such as humans and birds (17, 22). In a previous study (23), it was found that all fosA7-positive Salmonella isolates were susceptible to fosfomycin, whereas fosA7.5 detected in this study can confer high-level fosfomycin resistance (MIC ≥ 512 μg/mL) in E. coli. It is worth noting that as avians, pigeons can transmit the strains carrying the fosA7-like gene into other natural habitats, which seems to provide a pathway for the spread of resistance genes. The above analysis shows that fosA7 gene has appeared in food animals, birds, and environments where humans live, and pigeons might be considered a source or vector of resistant isolates posing a threat to public and animal health.
In this study, except for one strain that was resistant to only two antibiotics, all fosA/fosA3/fosA7.5-bearing Enterobacteriaceae isolates were MDR and displayed a high rate of resistance to ceftazidime, florfenicol, tetracycline, and ciprofloxacin. The high prevalence of drug resistance in fosfomycin-resistant strains may be related to the overuse of these drugs in farms. Furthermore, we also found that fosA3 or fosA7.5 was often coharbored with blaCTX-M, floR, and blaTEM in the same strain, similar to a previous report (24), which is likely to facilitate the dissemination and maintenance of fosA3 by coselection. However, the current study identified only 9 rmtB-producing isolates (9/52), which was in contrast to a prior study (25). In China, because of the widespread use of tetracycline, cephalosporins, aminoglycosides, and florfenicol as treatments or feed additives in animal husbandry, strains containing fosA-like genes have a high occurrence of other resistance genes (26). Therefore, limiting the use of antibiotics in animal agriculture may help prevent the spread of fosA-like genes in strains.
In this study, ERIC-PCR typing showed 6 unique clusters and 11 ERIC types for 52 fosA3-carrying E. coli isolates, which revealed genetic diversity. Moreover, some isolates had identical ERIC profiles, indicating dissemination from a similar origin. This result was consistent with a previous report that there was both clonal and horizontal transmission of these fosA3-positive E. coli (27). MLST analysis of 29 conjugable fosA3-positive E. coli isolates identified 15 STs, and ST115 was the most prevalent type, followed by ST156. However, ST115 and ST156 were previously found in ESBL-producing E. coli strains recovered from food and human samples (28, 29). MLST combined with ERIC-PCR analyses indicated that the 10 fosA7.5-positive E. coli isolates were mainly cloned among pigeons, which should arouse attention. At the same time, it demonstrated that the prevalence of fosfomycin-resistant strains has gradually increased, resulting in more serious problems of drug resistance.
The fosA gene was reported on conjugative plasmids or transposon Tn2921 of S. marcescens strains (20, 21) in which the encoded protein FosATn2921 is closely related to FosA, encoded on the chromosome of E. cloacae, indicating that fosA has been transferred between strains. All fosA-positive isolates in this study showed high levels of resistance to fosfomycin, but no fosA-carrying transconjugants were obtained, implying that fosA might be located on the chromosomes or nonconjugative plasmids of these Enterobacteriaceae isolates. Upon analysis of the genetic environment of fosA, a partial sequence similar to the transposon Tn2921 and E. cloacae ECNIH5 was found, which suggested that mobile elements or transposons were the primary reason for the extensive spread of fosA among Enterobacteriaceae.
Our findings showed that fosA3 was successfully transferred from donors to the recipient E. coli C600, implying that fosA3 could be horizontally transferred to different bacterial individuals. Furthermore, this work identified six genetic environments of fosA3, and fosA3 was frequently flanked by IS26, consistent with previous studies (30). Besides IS26, the different mobile elements identified in the regions surrounding fosA3 and other resistance genes by WGS analysis include IS91, IS4, ISVsa3, and IS1. These elements might play an important role in spreading antimicrobial resistance genes in Gram-negative bacteria (31). In short, the diversity of genetic contexts reflects the complexity of fosA3 transmission in E. coli. According to previous reports, the fosA3-carrying plasmids were mainly IncFII, IncN, and IncFIB plasmids (32). In this study, fosA3 was discovered on the conjugative IncFII plasmid. Additionally, the full sequence comparison analysis of plasmid showed that the IncFII plasmid in this study has high homology (>99%) with other IncFII plasmids carrying fosA3 from different sources, especially humans and chickens, suggesting that fosA3-bearing IncFII plasmids are widely present in animals and humans.
Contrary to previous reports (17), no fosA7.5-carryig transconjugants were obtained in this study. The fosA7.5 gene was located on the chromosomes of E. coli isolates belonging to ST602 and ST2599 and shared 100% similarity with fosA7.5WT. This study showed that fosA7.5 could confer resistance to fosfomycin, because of the amino acid difference between FosA7.5 found in E. coli and FosA7 first found in Salmonella serovar Heidelberg, which is a crucial factor for the fosA7.5 gene to show resistance to fosfomycin in E. coli. In this study, the isolates frequently contained insertion sequences (ISL3 and IS3) both upstream and downstream of fosA7.5. As previously reported (33), fosA7 alleles on the chromosomes could act as reservoirs of potential resistance genes, and they can be captured by mobile genetic elements to horizontally disseminate between different bacteria. In addition, fosA7.5-positive E. coli ST602 and ST2599 were found to be clonally transmitted, leading to an increased risk of drug resistance transmission to humans via the food chain, which could pose a serious threat to public health.
In conclusion, this study revealed a high prevalence and complex genetic environment of fosA-like genes in farm samples. Whether fosA-like genes are located on the chromosomes or plasmids of isolates, they may spread, mediated by mobile elements. The fosfomycin resistance gene is potentially transferred to the human body through the food chain, thus increasing the risk for human public health, and should be regularly monitored.
MATERIALS AND METHODS
Bacterial strains.
From September 2019 to December 2020, a total of 531 samples were collected from animals (chicken, pig, and pigeon) and their surroundings (sewage and soil) in farms in Guangxi Province, China. All samples were screened for the presence of fosfomycin-resistant isolates. Briefly, the samples were placed into LB broth and shaken at 37°C for approximately 16 to 18 h. Then, the fosfomycin-resistant isolates were selected on xylose-lysine-deoxycholate (XLD) agar plates (Enterobacteriaceae identification medium) containing 256 μg/mL fosfomycin. From each sample, only a single isolate of any one species was obtained. The strains were further identified using 16S rRNA sequencing (34), using primers described previously (F, AGAGTTTGATCATGGCTC; R, GGTTACCTTGTTACGACTT).
Identification of fosfomycin-resistant determinants and the coexisting resistance genes.
The existence of fosfomycin-modifying-enzyme genes (fosA3, fosA, fosC2, fosA7.5, and others) in all selected fosfomycin-resistant isolates was determined by PCR and sequencing (18), and the fosA7.5 primer was designed based on the sequence of fosA7 (17). The surrounding regions of the fosA-like genes were determined by PCR mapping and sequencing using previously published primers (18, 24). Furthermore, the florfenicol resistance gene floR, the 16S rRNA methyltransferase gene rmtB, the carbapenem resistance gene blaNDM, the ESBL genes blaCTX-M (groups 1, 2, 8, and 9) and blaTEM, and the plasmid-mediated AmpC lactamase gene blaCMY-2 were also identified using PCR and sequencing (35–38). All primers are listed in the supplemental material.
Antimicrobial susceptibility testing.
The MICs of 12 antibiotics (ceftazidime, florfenicol, chloramphenicol, erythromycin, tetracycline, ciprofloxacin, amikacin, meropenem, colistin, tigecycline, fosfomycin, and rifampicin) for the fosA-like gene-positive isolates were determined by the agar dilution method or broth microdilution method according to the CLSI (39). The MICs of fosfomycin were determined by the agar dilution method on Mueller-Hinton agar supplemented with 25 μg/mL glucose-6-phosphate (G-6-P), and the resistant breakpoints were recommended by the EUCAST in 2020 (40). E. coli ATCC 25922 was used as the control strain.
Conjugation assays and plasmid replicon typing.
The transferability of fosfomycin resistance genes was determined by broth mating method using the plasmid-free E. coli C600 strain (Rifr) as the recipient. Transconjugants were selected on MacConkey agar plates containing fosfomycin (100 μg/mL), G-6-P (25 μg/mL), and rifampicin (250 μg/mL) and finally confirmed by ERIC-PCR. When the conjugation experiments failed, E. coli DH5α was used as the recipient for transformation experiments. The transfer of the fosfomycin resistance genes (fosA, fosA3, or fosA7.5) was confirmed by PCR, and the MICs of transconjugants were also detected as described above. PCR-based replicon typing (PBRT) was used to screen the plasmid incompatibility groups for the fosA-like gene-positive isolates and their corresponding transconjugants (41). The primers are listed in the supplemental material.
MLST and ERIC-PCR.
The 29 conjugable fosA3-positive E. coli isolates and the 10 fosA7.5-harboring isolates were subjected to MLST analysis, which was performed as previously described (42). The STs were obtained from the MLST database website (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli). ERIC-PCR was carried out by using the primers ERIC-1 and ERIC-2 for fosA3-positive and fosA7.5-positive E. coli isolates (43). The isolated Enterobacteriaceae DNA samples were amplified in order to construct a computerized dendrogram, with the presence and absence of bands assumed to be 1 and 0, respectively. Following software processing, a matrix diagram of the binary number sequence was created and imported into NTSYS-pc (version 2.10) to perform the cluster analysis (44), which is based on the unweighted pair group method with arithmetic averages (UPGMA). Cluster were defined as being the same when the similarity between ERIC-PCR profiles was >80%.
Whole-genome sequencing and phylogenetic analysis.
Whole-genome sequencing of two representative E. coli isolates (fEC.1 and fEC.99-1) from pigeons was performed. The extracted total genomic DNA of isolates was sequenced using the Nanopore PromethION and Illumina NovaSeq PE150 sequencing platforms, and the reads were assembled using Unicycler software. The coding sequences of the genetic context surrounding fosA3 and fosA7.5 were analyzed using the ORF Finder program (www.ncbi.nlm.nih.gov/gorf/orfig.cgi), and annotation was performed using the RAST server (http://rast.nmpdr.org/). The plasmid replicon types and antibiotic resistance genes prediction were analyzed using tools found at http://pubmlst.org/plasmid/ and https://cge.cbs.dtu.dk/services/. Genome comparison analysis of plasmids was performed using Easyfig and BRIG. WGS information for E. coli isolates was downloaded from GenBank (Tables S5 and S6), and cgMLST was performed as described previously (45).
Cloning, expression, and functional verification of fosA7.5.
The fosA7.5 gene from E. coli fEC.1 was cloned into pET-28a(+) and was transferred into E. coli Top10 by heat shock. Transformants were selected on LB agar plates containing 100 μg/mL kanamycin. Then, the recombinant clones were identified by PCR and Sanger sequencing. The Top10 strain containing pET-28a(+)-fosA7.5 and the Top10 control strain were subjected to a fosfomycin resistance test to verify it’s functionality.
Data availability.
The fosA7.5-bearing chromosome sequences of fEc.1 and fEcg99-1 were submitted to NCBI with the accession numbers CP085638 and CP085637, respectively. The fosA3-bearing plasmid (pfEc.1-3) sequence was submitted with the accession number OK605583. The nucleotide sequences of fosA (types I, II, III, and IV), fosA3 (types I, II, IV, V, and VI), and fosA7.5 (type I) in this study have been deposited in GenBank under the accession numbers OM355477, OM289150, OM289151, OM355478, OM420281, OM355482, OM355480, OM355481, OM355483, and OM355479.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by The Key Research and Development Plan of Guangxi, China (AB19245037), Natural National Science Foundation of China (31760746), and the Major R&D Project of Nanning Qingxiu District (2020005).
Xiaoxiao Zhang and Mingxiang Ma analyzed and interpreted the data. Yiqin Huang, Yajing Qian, Yuxiao Tan, Yujie Lu, Yumeng Cheng, and Xin Zhong performed the experiments and collected the data. Yunqiao Yang contributed to the revision of the article. Hongbin Si designed this work. All authors agreed on and approved the final manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Hongbin Si, Email: shb2009@gxu.edu.cn.
Thomas G. Denes, University of Tennessee
Alexa Cohn, Cornell University.
REFERENCES
- 1.Ruiz Ramos J, Salavert Lletí M. 2019. Fosfomycin in infections caused by multidrug-resistant Gram-negative pathogens. Rev Esp Quimioter 32(Suppl 1):45–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Bi W, Li B, Song J, Hong Y, Zhang X, Liu H, Lu H, Zhou T, Cao J. 2017. Antimicrobial susceptibility and mechanisms of fosfomycin resistance in extended-spectrum β-lactamase-producing Escherichia coli strains from urinary tract infections in Wenzhou, China. Int J Antimicrob Agents 50:29–34. doi: 10.1016/j.ijantimicag.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Huang L, Cao M, Hu Y, Zhang R, Xiao Y, Chen G. 2021. Prevalence and mechanisms of fosfomycin resistance among KPC-producing Klebsiella pneumoniae clinical isolates in China. Int J Antimicrob Agents 57:106226. doi: 10.1016/j.ijantimicag.2020.106226. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Wang D, Ding Y, Zhang L, Li X. 2019. Molecular epidemiology of plasmid-mediated fosfomycin resistance gene determinants in Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae isolates in China. Microb Drug Resist 25:251–257. doi: 10.1089/mdr.2018.0137. [DOI] [PubMed] [Google Scholar]
- 5.Walkty A, Karlowsky JA, Baxter MR, Adam HJ, Alexander D, Bay DC, Boyd D, McCracken M, Mulvey MR, Zhanel GG. 2020. Fosfomycin resistance mediated by fos genes remains rare among extended-spectrum beta-lactamase-producing Escherichia coli clinical isolates recovered from the urine of patients evaluated at Canadian hospitals (CANWARD, 2007–2017). Diagn Microbiol Infect Dis 96:114962. doi: 10.1016/j.diagmicrobio.2019.114962. [DOI] [PubMed] [Google Scholar]
- 6.Yang T-Y, Lu P-L, Tseng S-P. 2019. Update on fosfomycin-modified genes in Enterobacteriaceae. J Microbiol Immunol Infect 52:9–21. doi: 10.1016/j.jmii.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Lee S-Y, Park Y-J, Yu JK, Jung S, Kim Y, Jeong SH, Arakawa Y. 2012. Prevalence of acquired fosfomycin resistance among extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J Antimicrob Chemother 67:2843–2847. doi: 10.1093/jac/dks319. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Lin Q, Zhou Q, Lv L, Wan M, Gao X, Wang C, Liu J-H. 2020. Identification of fosA10, a novel plasmid-mediated fosfomycin resistance gene of Klebsiella pneumoniae origin, in Escherichia coli. Infect Drug Resist 13:1273–1279. doi: 10.2147/IDR.S251360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou J, Yang X, Zeng Z, Lv L, Yang T, Lin D, Liu J-H. 2013. Detection of the plasmid-encoded fosfomycin resistance gene fosA3 in Escherichia coli of food-animal origin. J Antimicrob Chemother 68:766–770. doi: 10.1093/jac/dks465. [DOI] [PubMed] [Google Scholar]
- 10.Hou J, Huang X, Deng Y, He L, Yang T, Zeng Z, Chen Z, Liu J-H. 2012. Dissemination of the fosfomycin resistance gene fosA3 with CTX-M β-lactamase genes and rmtB carried on IncFII plasmids among Escherichia coli isolates from pets in China. Antimicrob Agents Chemother 56:2135–2138. doi: 10.1128/AAC.05104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Ma Z-B, Zeng Z-L, Yang X-W, Huang Y, Liu J-H. 2017. The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zool Res 38:55–80. doi: 10.24272/j.issn.2095-8137.2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehman MA, Yin X, Persaud-Lachhman MG, Diarra MS. 2017. First detection of a fosfomycin resistance gene, fosA7, in Salmonella enterica serovar Heidelberg isolated from broiler chickens. Antimicrob Agents Chemother 61:e00410-17. doi: 10.1128/AAC.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehman MA, Hasted T-L, Persaud-Lachhman MG, Yin X, Carrillo C, Diarra MS. 2019. Genome analysis and multiplex PCR method for the molecular detection of coresistance to cephalosporins and fosfomycin in Salmonella enterica serovar Heidelberg. J Food Prot 82:1938–1949. doi: 10.4315/0362-028X.JFP-19-205. [DOI] [PubMed] [Google Scholar]
- 15.Hua M, Huang W, Chen A, Rehmet M, Jin C, Huang Z. 2020. Comparison of antimicrobial resistance detected in environmental and clinical isolates from historical data for the US. Biomed Res Int 2020:4254530. doi: 10.1155/2020/4254530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Y, Hu B, Bai X, Yang X, Cao L, Liu Q, Sun H, Li J, Zhang J, Jin D, Xiong Y. 2021. Antimicrobial resistance of non-O157 Shiga toxin-producing Escherichia coli isolated from humans and domestic animals. Antibiotics (Basel) 10:74. doi: 10.3390/antibiotics10010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milner KA, Bay DC, Alexander D, Walkty A, Karlowsky JA, Mulvey MR, Sharma MK, Zhanel GG. 2020. Identification and characterization of a novel FosA7 member from fosfomycin-resistant Escherichia coli clinical isolates from Canadian hospitals. Antimicrob Agents Chemother 65:e00865-20. doi: 10.1128/AAC.00865-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Ou B, Zhang M, Chou C-H, Chang S-K, Zhu G. 2021. Coexistence of fosfomycin resistance determinant fosA and fosA3 in Enterobacter cloacae isolated from pets with urinary tract infection in Taiwan. Microb Drug Resist 27:415–423. doi: 10.1089/mdr.2020.0077. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Tian A, Wang J, Zhu Y, Xie Z, Zhang R, Jiang S. 2022. Occurrence and molecular epidemiology of fosA3-bearing Escherichia coli from ducks in Shandong province of China. Poult Sci 101:101620. doi: 10.1016/j.psj.2021.101620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendoza C, Garcia JM, Llaneza J, Mendez FJ, Hardisson C, Ortiz JM. 1980. Plasmid-determined resistance to fosfomycin in Serratia marcescens. Antimicrob Agents Chemother 18:215–219. doi: 10.1128/AAC.18.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Lobo JM, Ortiz JM. 1982. Tn292l, a transposon encoding fosfomycin resistance. J Bacteriol 151:477–479. doi: 10.1128/jb.151.1.477-479.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skarżyńska M, Zaja C M, Bomba A, Bocian Ł, Kozdruń W, Polak M, Wia Cek J, Wasyl D. 2021. Antimicrobial resistance glides in the sky–free-living birds as a reservoir of resistant Escherichia coli with zoonotic potential. Front Microbiol 12:656223. doi: 10.3389/fmicb.2021.656223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Wang Y, Wang Z-Y, Wu H, Mei C-Y, Shen P-C, Pan Z-M, Jiao X. 2021. Chromosomally located fosA7 in Salmonella isolates from China. Front Microbiol 12:781306. doi: 10.3389/fmicb.2021.781306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W, Men S, Kong L, Ma S, Yang Y, Wang Y, Yuan Q, Cheng G, Zou W, Wang H. 2017. Prevalence of plasmid-mediated fosfomycin resistance gene fosA3 among CTX-M-producing Escherichia coli isolates from chickens in China. Foodborne Pathog Dis 14:210–218. doi: 10.1089/fpd.2016.2230. [DOI] [PubMed] [Google Scholar]
- 25.Xiang D-R, Li J-J, Sheng Z-K, Yu H-Y, Deng M, Bi S, Hu F-S, Chen W, Xue X-W, Zhou Z-B, Doi Y, Sheng J-F, Li L-J. 2015. Complete sequence of a novel IncR-F33:A-:B- plasmid, pKP1034, harboring fosA3, blaKPC-2, blaCTX-M-65, blaSHV-12, and rmtB from an epidemic Klebsiella pneumoniae sequence type 11 strain in China. Antimicrob Agents Chemother 60:1343–1348. doi: 10.1128/AAC.01488-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Liu W, Liu Y, Wang J, Lv L, Chen X, He D, Yang T, Hou J, Tan Y, Xing L, Zeng Z, Liu J-H. 2014. F33:A-:B-, IncHI2/ST3, and IncI1/ST71 plasmids drive the dissemination of fosA3 and bla CTX-M-55/-14/-65 in Escherichia coli from chickens in China. Front Microbiol 5:688. doi: 10.3389/fmicb.2014.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han L, Lu X-Q, Liu X-W, Liao M-N, Sun R-Y, Xie Y, Liao X-P, Liu Y-H, Sun J, Zhang R-M. 2021. Molecular epidemiology of fosfomycin resistant E. coli from a pigeon farm in China. Antibiotics 10:777. doi: 10.3390/antibiotics10070777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie M, Lin D, Chen K, Chan EWC, Yao W, Chen S. 2016. Molecular characterization of Escherichia coli strains isolated from retail meat that harbor blaCTX-M and fosA3 genes. Antimicrob Agents Chemother 60:2450–2455. doi: 10.1128/AAC.03101-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortés P, Blanc V, Mora A, Dahbi G, Blanco JE, Blanco M, López C, Andreu A, Navarro F, Alonso MP, Bou G, Blanco J, Llagostera M. 2010. Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in Spain. Appl Environ Microbiol 76:2799–2805. doi: 10.1128/AEM.02421-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He L, Partridge SR, Yang X, Hou J, Deng Y, Yao Q, Zeng Z, Chen Z, Liu J-H. 2013. Complete nucleotide sequence of pHN7A8, an F33:A-:B-type epidemic plasmid carrying blaCTX-M-65, fosA3 and rmtB from China. J Antimicrob Chemother 68:46–50. doi: 10.1093/jac/dks369. [DOI] [PubMed] [Google Scholar]
- 31.Partridge SR, Kwong SM, Firth N, Jensen SO. 2018. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 31:e00088-17. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X-M, Dong Z, Schwarz S, Zhu Y, Hua X, Zhang Y, Liu S, Zhang W-J. 2017. Plasmids of diverse Inc groups disseminate the fosfomycin resistance gene fosA3 among Escherichia coli isolates from pigs, chickens, and dairy cows in northeast China. Antimicrob Agents Chemother 61:e00859-17. doi: 10.1128/AAC.00859-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partridge SR. 2011. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol Rev 35:820–855. doi: 10.1111/j.1574-6976.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 34.Walsh F, Duffy B. 2013. The culturable soil antibiotic resistome: a community of multi-drug resistant bacteria. PLoS One 8:e65567. doi: 10.1371/journal.pone.0065567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv L, Huang X, Wang J, Huang Y, Gao X, Liu Y, Zhou Q, Zhang Q, Yang J, Guo J-Y, Liu J-H. 2020. Multiple plasmid vectors mediate the spread of fosA3 in extended-spectrum-β-lactamase-producing Enterobacterales isolates from retail vegetables in China. mSphere 5:e00507-20. doi: 10.1128/mSphere.00507-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maynard C, Fairbrother JM, Bekal S, Sanschagrin F, Levesque RC, Brousseau R, Masson L, Larivière S, Harel J. 2003. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149:K91 isolates obtained over a 23-year period from pigs. Antimicrob Agents Chemother 47:3214–3221. doi: 10.1128/AAC.47.10.3214-3221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tagg KA, Iredell JR, Partridge SR. 2014. Complete sequencing of IncI1 sequence type 2 plasmid pJIE512b indicates mobilization of blaCMY-2 from an IncA/C plasmid. Antimicrob Agents Chemother 58:4949–4952. doi: 10.1128/AAC.02773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Chen Z-L, Liu J-H, Zeng Z-L, Ma J-Y, Jiang H-X. 2007. Emergence of RmtB methylase-producing Escherichia coli and Enterobacter cloacae isolates from pigs in China. J Antimicrob Chemother 59:880–885. doi: 10.1093/jac/dkm065. [DOI] [PubMed] [Google Scholar]
- 39.CLSI. 2018. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. CLSI standard VET01. Clinical and Laboratory Standards Institute, Wayne, PA, USA. [Google Scholar]
- 40.EUCAST. 2020. Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint-t00les/v_10.0_Breakpoint-T00les.pdf.
- 41.Johnson TJ, Wannemuehler YM, Johnson SJ, Logue CM, White DG, Doetkott C, Nolan LK. 2007. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl Environ Microbiol 73:1976–1983. doi: 10.1128/AEM.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gauthier L, Dortet L, Cotellon G, Creton E, Cuzon G, Ponties V, Bonnin RA, Naas T. 2018. Diversity of carbapenemase-producing Escherichia coli isolates in France in 2012–2013. Antimicrob Agents Chemother 62:e00266-18. doi: 10.1128/AAC.00266-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moosavian M, Emam N. 2019. The first report of emerging mobilized colistin-resistance (mcr) genes and ERIC-PCR typing in Escherichia coli and Klebsiella pneumoniae clinical isolates in southwest Iran. Infect Drug Resist 12:1001–1010. doi: 10.2147/IDR.S192597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan W, Chai TJ, Miao ZM. 2010. ERIC-PCR identification of the spread of airborne Escherichia coli in pig houses. Sci Total Environ 408:1446–1450. doi: 10.1016/j.scitotenv.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Feng Y, Zou S, Chen H, Yu Y, Ruan Z. 2021. BacWGSTdb 2.0: a one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res 49:D644–D650. doi: 10.1093/nar/gkaa821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.00545-22-s0001.pdf, PDF file, 0.7 MB (769KB, pdf)
Data Availability Statement
The fosA7.5-bearing chromosome sequences of fEc.1 and fEcg99-1 were submitted to NCBI with the accession numbers CP085638 and CP085637, respectively. The fosA3-bearing plasmid (pfEc.1-3) sequence was submitted with the accession number OK605583. The nucleotide sequences of fosA (types I, II, III, and IV), fosA3 (types I, II, IV, V, and VI), and fosA7.5 (type I) in this study have been deposited in GenBank under the accession numbers OM355477, OM289150, OM289151, OM355478, OM420281, OM355482, OM355480, OM355481, OM355483, and OM355479.