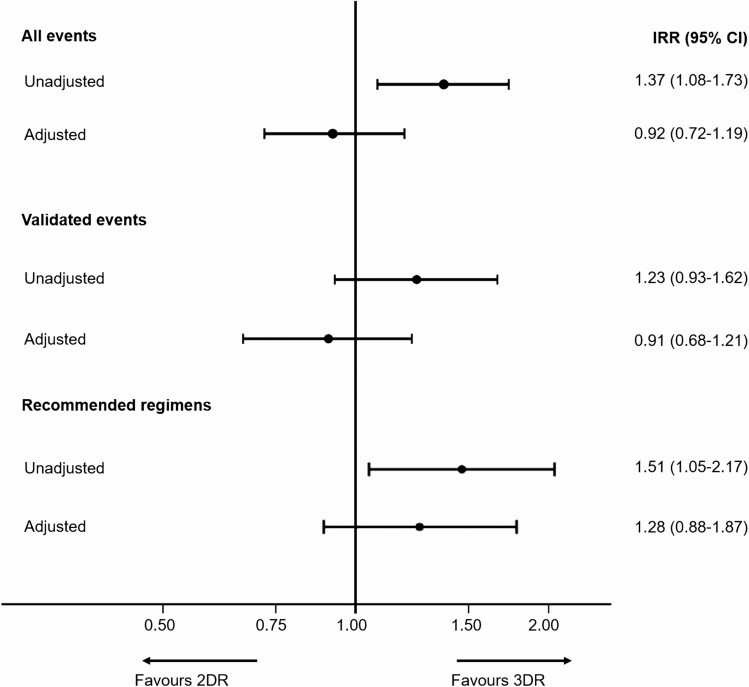
Figure 3.
IRR comparing events on 2DRs vs 3DRs. All events and validated events—adjusted analyses adjusted for age, gender, ethnicity, body mass index, smoking status, human immunodeficiency virus (HIV) risk group, HIV viral load at regimen start, nadir CD4 count, CD4 cell count at regimen start, viral hepatitis C, viral hepatitis B, prior hypertension, prior diabetes, prior AIDS-defining event (excluding cancer), prior AIDS cancer, prior non-AIDS cancer, prior end stage liver disease, prior cardiovascular disease, prior fracture, prior chronic kidney disease, prior dyslipidemia, and number of drugs previously exposed to. Recommended regimens—adjusted analysis adjusted for age, gender, ethnicity, smoking status,CD4 cell count at regimen start, viral hepatitis C, prior AIDS-defining event (excluding cancer), prior non-AIDS cancer, prior cardiovascular disease, prior chronic kidney disease, number of drugs previously exposed to. Recommended regimens included 2DRs: dolutegravir (DTG) plus rilpivirine (RPV), DTG plus lamivudine (3TC), boosted atazanavir (ATV/b) plus 3TC, darunavir (DRV) plus 3TC, DRV plus RPV; 3DRs: DTG or RPV or ATV/b or DRV plus 2 nucleoside reverse transcriptase inhibitors. Abbreviations: 2DR, 2-drug regimen; 3DR, 3-drug regimen; CI, confidence interval; IRR, incidence rate ratio.