Figure 2.
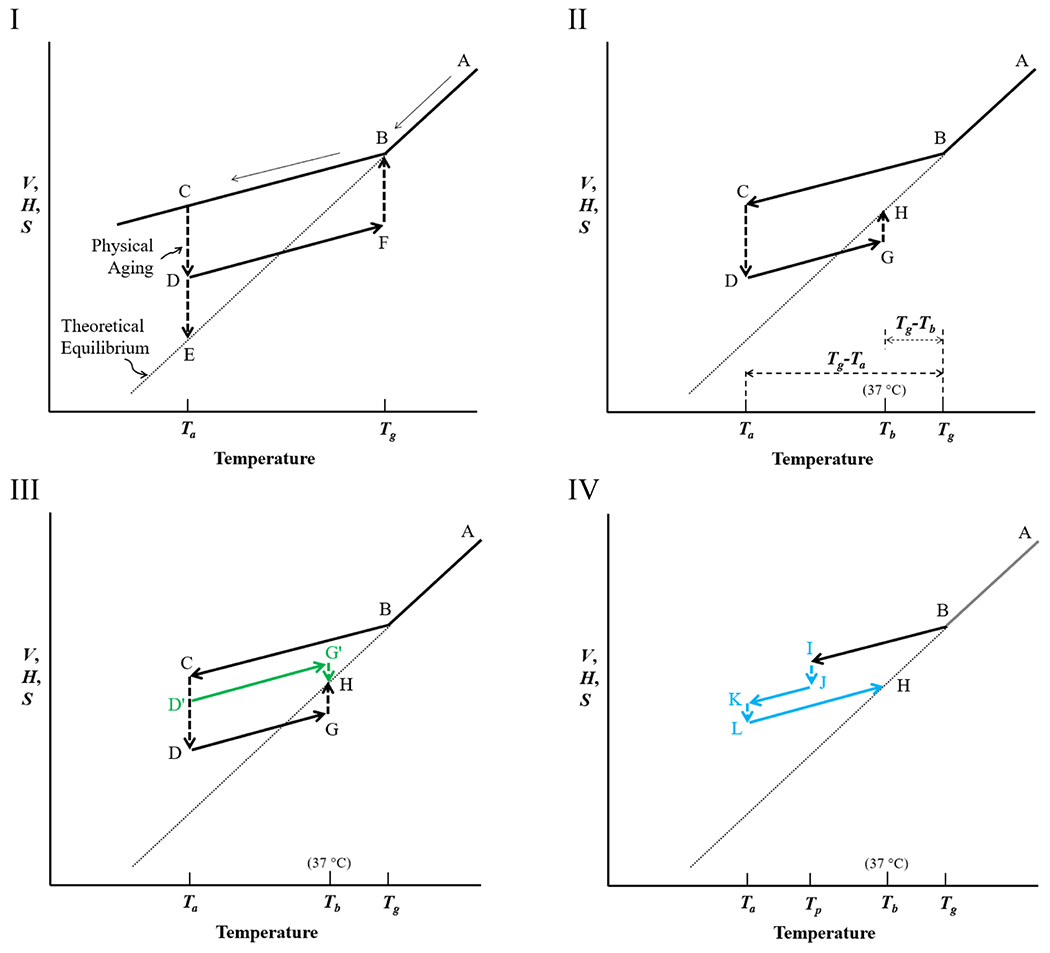
Volume (V) (or enthalpy (H), or entropy (S)) as a function of temperature for PLGA microparticles. (Temperature can be replaced with solvent fraction). (I) As the equilibrium liquid (A) is cooled, the liquid becomes glassy (B) at Tg. At the annealing temperature, Ta, the volume of the system (C) is lowered (D) to reach an equilibrium glassy state (E). As the aged glass (D) is heated to the Tg, the volume increases to F and then to B, an equilibrium rubbery state. (II) When the aged glass (D) is heated to 37 °C, body temperature, Tb, the volume rises to G and reaches an equilibrium state (H). The times for physical aging (C → D) and for reaching equilibrium at Tb (G → H) depend on the magnitude of Tg-Ta, and Tg-Tb, respectively. (III) At Ta, physical aging occurs to different extents, D′ or D, depending on the aging time. When the temperature is raised to Tb, G’ or G reaches the equilibrium state H. (IV) The prepared glassy PLGA microparticles (I) may be exposed again to a poor solvent (e.g., ethanol) at the temperature of preparation (Tp), and the volume is reduced (J), then dried again, and stored at Ta (K). The glassy PLGA may undergo further aging to L before the temperature is increased again to Tb.
