Abstract
Objective
This study aimed to describe the prevalence of Epstein-Barr virus (EBV)-DNA among children in Suzhou, and to explore the association between plasma EBV load and disease diagnosis.
Methods
All children admitted to the Children's Hospital of Soochow University between January 2018 and September 2020 and subjected to the plasma EBV-DNA assay were included. The authors retrospectively collected demographic and discharge diagnostic information of the participants, and ascribed the disease distribution characteristics of children with positive plasma EBV-DNA by age and viral load.
Results
A total of 38,175 patients underwent plasma EBV-DNA PCR assay, of which 2786 (7.3%) had EBV-DNA in their plasma. Children aged 3–4 years had a high prevalence of EBV infection. Plasma EBV positivity was common with infectious mononucleosis (IM, 40.0%), respiratory infection (20.1%), atypical EBV infection (14.2%), acute leukemia (6.4%), hemophagocytic lymphohistiocytosis (HLH, 4.8%), and idiopathic thrombocytopenic purpura (ITP, 2.9%). With increasing age, plasma EBV positivity was more common in children with IM and atypical EBV infection. However, an inverse correlation was observed in children with respiratory infections and ITP. High levels of EBV loads were more likely to occur in HLH, IM, and atypical EBV infection, especially in HLH. However, lower viral loads were found in respiratory infection and acute leukemia.
Conclusions
This is a large sample study that revealed the prevalence of plasma EBV-DNA levels in children of various ages and presenting illnesses.
Keywords: Epstein‐Barr virus, Child, Prevalence
Introduction
Epstein-Barr virus (EBV) was first discovered in cells isolated from Burkitt's lymphoma by Epstein and Barr in 1964 and was later classified as human herpesvirus 4 (HHV4).1 It is highly prevalent worldwide, with more than 90% of the world's adults being infected with the virus.2 In developed countries such as Europe and the United States, people develop primary infections in late adolescence. However, people experience primary infection much earlier in developing areas.3 Similar to other herpesviruses, following primary infection, EBV has a latency phase where it infects oropharyngeal epithelial cells, enters the blood, binds to CD21 receptors on B lymphocytes, and persists for life in a latent state.4 EBV infection is associated with many diseases, including infectious mononucleosis (IM),5 Burkitt lymphoma, Hodgkin lymphoma, nasopharyngeal carcinoma (NPC), and gastric carcinoma.3 A previous study6 has shown that the most common acute presentation of EBV infection is a febrile viral upper respiratory illness. In addition, a literature search7 showed that EBV infection was also associated with head and neck cancer, breast cancer, lymphoproliferative disorders, EBV-HLH, vitamin D deficiency, systemic lupus erythematosus, chronic fatigue syndrome, rheumatoid arthritis (RA), thyroid disorders, multiple sclerosis (MS), and other autoimmune disorders.
Heterophile assays may be falsely negative in young children, and EBV-specific antibody tests are accurate. At present, the diagnosis of EBV infection in children mainly relies on serological and molecular biological tests. Moreover, molecular biological assays have been shown to be a good supplement to serological testing.8 The measurement of EBV using real-time polymerase chain reaction (RT-PCR) has become an essential tool in the diagnosis and monitoring of EBV infection.9 In addition, plasma EBV-DNA has been used as a marker of active EBV infection.
This study used a large number of samples to describe the epidemiological characteristics of EBV-DNA in children, such as the patients’ age, gender, and diseases, consequently providing more baseline information to clinicians.
Methods
Patients and samples
All children admitted to the Children's Hospital of Soochow University between January 1, 2018, and September 30, 2020, including outpatients and inpatients, and who were subjected to plasma EB viral nucleic acid assays, were included in this study. The authors retrospectively collected demographic and diagnostic information (discharge diagnosis) and analyzed the epidemiological features of children with positive plasma EBV-DNA at different ages and illnesses. A total of 38,175 patients (23,027 boys and 15,148 girls), with ages ranging from 0 to 16 years, were enrolled in this retrospective study.
Laboratory testing
Plasma EBV-DNA PCR assay: Peripheral blood (1 mL) was obtained from the admitted patient and immediately centrifuged for examination; then, 10 μL of plasma were mixed with DNA extract (Shengxiang Biotechnology Co., Ltd, Hunan, China). Next, 40 μL of the PCR mixture was added, and the mixture was centrifuged for PCR. PCR was performed on a LightCycler 480II instrument (Roche, Basel, Switzerland) under the following conditions: 50°C for 2 min and 94 °C for 2 min, followed by 45 cycles of 94 °C for 5 s, 57 °C for 30 s, and 25 °C for 10 s. For each test, a negative, critical, positive, and four positive quantity controls were used. DNA viral loads were calculated by comparing the cycle threshold (Ct) of the specimens to the standard curve. EBV negativity was defined as the Ct value of the negative control, while positivity was defined as a Ct value ≤ 39 (DNA copy number > 400 copies/mL).
Diagnostic criteria for atypical EBV infection
In this study, atypical EBV infection was defined as the onset of fever and/or elevated atypical lymphocytes in the peripheral blood without target organ damage.
Statistical analysis
Data are presented as n (%) or median (interquartile range). All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA). Continuous data were analyzed using the Student's t-test. Categorical variables were compared using the chi-square test. The Kruskal-Wallis rank-sum test was used for continuous variables with skewed distributions. Logistic regression analysis was used to determine odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was set at p < 0.05.
Ethics approval and consent to participate
This study adhered to the ethical principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Children's Hospital of Soochow University (No.2019KS004). For the presented retrospective data, the requirement to obtain informed consent was waived in accordance with the Ethics Committee's vote.
Results
Patient characteristics
The research involved 38,175 children who were subjected to the plasma EBV-DNA assay, including 35,389 patients with negative EBV DNA tests and 2786 patients with positive EBV DNA tests. The EBV DNA positivity rate was 7.3%. The positive rate of EBV DNA in 4940 outpatients and 33,235 inpatients was 13.2% (650) and 6.4% (2136), respectively (p < 0.05). The median age of all enrolled children was 4.58 years (interquartile range 2.33–7.75 years), with a male to female ratio of 1.52.
The prevalence of plasma EBV-DNA in children of different ages
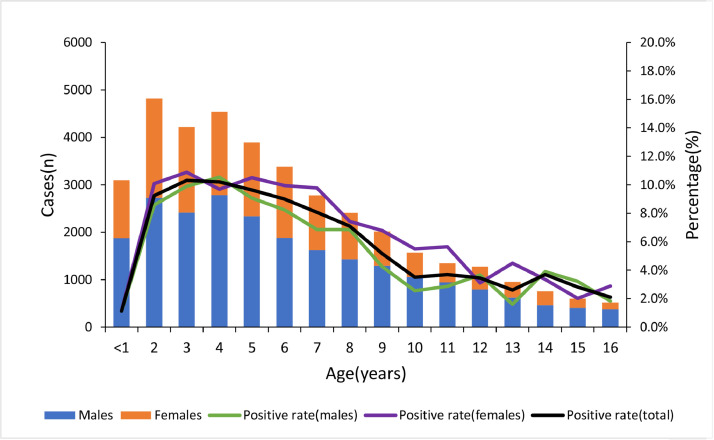
As shown in Fig. 1 and Supplemental Table 1, the positive rate of EBV-DNA was the lowest at the 0–1 year age group, which increased gradually in the older groups and decreased slightly after reaching the peak at 3–4 years of age. In addition, there was no significant difference in the positive rate of EBV-DNA between males and females at different ages (t = 0.980, p = 0.335).
Figure 1.
Positive rate of plasma EBV-DNA in different age and sex groups.
The proportion of differential disease diagnosis in children with plasma EBV-DNA
In the 2136 EBV-DNA positive inpatients, the six most common disease diagnoses were IM (40.0%), respiratory diseases (20.1%), atypical EBV infection (14.2%), acute leukemia (6.4%), hemophagocytic lymphohistiocytosis (HLH, 4.8%), and ITP (2.9%) (Table 1). There were significant differences in the age and viral loads among these six diseases (p < 0.05). Children with respiratory infection and ITP were younger than those with IM, atypical EBV infection, and acute leukemia (p < 0.05). The EB viral load in children with HLH was higher, and the EB viral load was lower in patients with a respiratory infection and acute leukemia (p < 0.05).
Table 1.
Plasma EBV loads in patients with various diseases.
| Disease | n | Gender (male) | Age (year) | EBV-DNA lg (copies/mL) |
|---|---|---|---|---|
| IM | 854 | 482 (56.4%) | 4.3 (2.8–6.3) | 3.5 (3.1–4.0) |
| Atypical EBV infection | 304 | 148 (48.7%) | 4.9 (2.8–7.1) | 3.6 (3.1–4.2) |
| Respiratory infection | 430 | 265 (61.6%) | 3.2 (1.8–4.9) | 3.2 (2.9–3.6) |
| Acute leukemia | 137 | 94 (68.6%) | 4.7 (2.5–8.7) | 3.2 (2.8–3.7) |
| HLH | 103 | 27 (26.2%) | 4.1 (1.9–5.8) | 3.9 (3.2–5.2) |
| ITP | 61 | 25 (41.0%) | 2.3 (1.4–3.6) | 3.2 (2.9–3.9) |
| p | < 0.001 | < 0.001 | < 0.001 |
IM, infectious mononucleosis; HLH, hemophagocytic lymphohistiocytosis; ITP, idiopathic thrombocytopenic purpura.
The data are reported as n (%) or median [interquartile range].
The association of plasma EB viral load and disease diagnosis
As shown in Table 2, according to the age of the children with positive EBV-DNA test, they were divided into four groups: ≤ 1 year, 1–3 years, 3–7 years, and > 7 years. In children with plasma EBV-DNA, IM had a high prevalence in children aged 3–7 years (adjusted Odds ratio, aOR 15.2, 95%CI6.3–36.6) and atypical EBV infection was more common in children aged >7 years (aOR 3.4, 95%CI1.1–10.1). However, the authors observed a striking association between lower prevalence and increasing age in patients with a respiratory infection and ITP (p < 0.01). In the children with a high level of viral load, there was a higher prevalence of IM (aOR 2.0, 95%CI1.5–2.5), atypical EBV infection (aOR 2.3, 95%CI1.6–3.2), and EBV-HLH (aOR 3.1, 95%CI1.8–5.4), and lower prevalence of respiratory infection (aOR 0.3, 95%CI0.2–0.4) and acute leukemia (aOR 0.4, 95%CI0.3–0.8) (Table 3).
Table 2.
Multivariable-adjusted association of age and diseases in children with positive plasma EBV-DNA.
| Age (years) | Total | IM |
Atypical EBV infection |
Respiratory infection |
Acute leukemia |
HLH |
ITP |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | Aor (95%CI) | p | n | aOR (95%CI) | p | n | aOR (95%CI) | p | n | aOR (95%CI) | p | n | aOR (95%CI) | p | n | aOR (95%CI) | p | |
| ≤ 1 | 48 | 7 | 1 (reference) | 4 | 1 (reference) | 17 | 1 (reference) | 3 | 1 (reference) | 2 | 1 (reference) | 6 | 1 (reference) | ||||||
| 1–3 | 687 | 230 | 5.6 (2.3–13.5) | < 0.01 | 78 | 1.7 (0.6–5.1) | 0.32 | 190 | 0.8 (0.4–1.7) | 0.56 | 38 | 1.0 (0.3–3.6) | 0.97 | 43 | 1.8 (0.4–8.0) | 0.42 | 30 | 0.4 (0.1–0.9) | 0.03 |
| 3–7 | 1012 | 464 | 15.2 (6.3–36.6) | < 0.01 | 146 | 2.2 (0.8–6.4) | 0.15 | 182 | 0.3 (0.2–0.7) | < 0.01 | 52 | 0.9 (0.3–3.0) | 0.85 | 40 | 1.0 (0.2–4.5) | 0.96 | 23 | 0.2 (0.1–0.4) | < 0.01 |
| > 7 | 389 | 153 | 7.6 (3.1–18.8) | < 0.01 | 76 | 3.4 (1.1–10.1) | 0.03 | 41 | 0.2 (0.1–0.4) | < 0.01 | 44 | 2.2 (0.6–7.7) | 0.21 | 18 | 1.2 (0.3–5.6) | 0.79 | 2 | 0.04 (0.01–0.19) | < 0.01 |
IM, infectious mononucleosis; HLH, hemophagocytic lymphohistiocytosis; ITP, idiopathic thrombocytopenic purpura; aOR, odds ratio adjusted for gender; CI, confidence interval.
Table 3.
Multivariable-adjusted association of viral loads and diseases.
| Viral load lg(copies/mL) | Total | IM |
Atypical EBV infection |
Respiratory infection |
Acute leukemia |
EBV-HLH |
ITP |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | aOR (95%CI) | p | n | aOR (95%CI) | p | n | aOR (95%CI) | p | n | aOR (95%CI) | p | n | aOR (95%CI) | p | n | aOR (95%CI) | p | |
| 2.0–3.0 | 538 | 161 | 1 (reference) | 55 | 1 (reference) | 156 | 1 (reference) | 48 | 1 (reference) | 19 | 1 (reference) | 20 | 1 (reference) | ||||||
| 3.0–4.0 | 1115 | 473 | 1.7 (1.4–2.1) | < 0.01 | 150 | 1.4 (1.0–1.9) | 0.06 | 228 | 0.6 (0.5–0.8) | < 0.01 | 69 | 0.7 (0.5–1.0) | 0.05 | 35 | 0.9 (0.5–1.6) | 0.67 | 29 | 0.7 (0.4–1.3) | 0.23 |
| > 4.0 | 483 | 220 | 2.0 (1.5–2.5) | < 0.01 | 99 | 2.3 (1.6–3.2) | < 0.01 | 46 | 0.3 (0.2–0.4) | < 0.01 | 20 | 0.4 (0.3–0.8) | < 0.01 | 49 | 3.1 (1.8–5.4) | < 0.01 | 12 | 0.7 (0.3–1.4) | 0.32 |
IM, infectious mononucleosis; HLH, hemophagocytic lymphohistiocytosis; ITP, idiopathic thrombocytopenic purpura; aOR, odds ratio adjusted for gender and age; CI, confidence interval.
Discussion
EBV, a γ herpesvirus, is widespread worldwide, and evidence of the previous infection is present in adults, with more than 90% showing EBV-IgG positivity.2 In this study, the positive detection rate of plasma EBV-DNA in children aged 0–16 years was 7.3%, which was lower than the 11.3% to 14.3% in previous studies.10,11 An acute infection rate of 6.8% has been reported.12 In addition, this study found that the positive rate of EBV in outpatients was significantly higher than that in inpatients. The reason is that most of the outpatients are returning patients who have been infected with EBV, and plasma EBV could very well reflect the reactivation of a previous infection. The age distribution of EBV-DNA positive rate shows that it was the lowest at 0–1 year, then increased gradually in the older groups and decreased slightly after reaching the peak at 3–4 years old, which is considered to be related to the primary EBV infection mainly occurring in Chinese children at 2–4 years old.13
It has been demonstrated that EBV was associated with a variety of diseases such as IM, respiratory tract infection, asymptomatic infection, malignant lymphoma, nasopharyngeal carcinoma, aplastic anemia, HLH, immune dysfunction, chronic fatigue syndrome, and autoimmune diseases.7,10,13 In this study, EBV activation was observed in a variety of diseases, such as IM, respiratory diseases, atypical EBV infection, acute leukemia, HLH, and ITP. Furthermore, EBV activation in infants and young children mainly occurred with respiratory diseases and ITP, and in older children, it mainly occurred with IM and EBV atypical infections. However, previous studies14,15 have shown that younger children are more likely to have atypical symptoms. This is considered to be related to the fact that atypical symptoms in young children are too mild to attract parents’ attention.
This study also demonstrated the prevalence of plasma EBV DNA levels in children with various illnesses. A high EB viral load was more common with IM, atypical EBV infection, and HLH, while a low viral load was more common with respiratory diseases and acute leukemia. Previous studies have shown that EBV load is related to the severity of the disease,16 and EBV-HLH had a higher viral load than the other EBV-related diseases.10,17,18 This study showed that there was no obvious correlation between disease severity and viral load. The reason was that the plasma EBV-DNA test could very well reflect the reactivation of a previous infection rather than a primary infection,6 and causality cannot be attributed to EBV.
The present study is the first large sample investigation to describe the prevalence of plasma EBV DNA levels in children of various ages and presenting illnesses. This revealed that EBV activation was related to age and viral load in different diseases and provided clinicians with more baseline information. The present study has some limitations. First, this was a retrospective study; second, it lacked a serological assay for analysis; third, it did not account for unmeasured potential confounding factors, such as coinfection with other pathogens.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request (2231365607@qq.com; shiting0915@126.com).
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jped.2021.05.006.
Appendix. Supplementary materials
References
- 1.de Thé G., Day N., Geser A., Ho J.H., Simons M.J., Sohier R., et al. Epidemiology of the Epstein–Barr virus infection and associated tumors in man. Bibl Haematol. 1975;43:216–220. doi: 10.1159/000399133. [DOI] [PubMed] [Google Scholar]
- 2.Thompson M.P., Kurzrock R. Epstein–Barr virus and cancer. Clin Cancer Res. 2004;10:803–821. doi: 10.1158/1078-0432.ccr-0670-3. [DOI] [PubMed] [Google Scholar]
- 3.Balfour Jr, HH, Verghese P. Primary Epstein–Barr virus infection: impact of age at acquisition, coinfection, and viral load. J Infect Dis. 2013;207:1787–1789. doi: 10.1093/infdis/jit096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young L.S., Rickinson A.B. Epstein–Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 5.Evans A.S. Infectious mononucleosis and related syndromes. Am J Med Sci. 1978;276:325–339. doi: 10.1097/00000441-197811000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Nowalk A., Green M. Epstein–Barr virus. Microbiol Spectr. 2016;4:127–134. doi: 10.1128/microbiolspec.DMIH2-0011-2015. [DOI] [PubMed] [Google Scholar]
- 7.Fugl A., Andersen C.L. Epstein–Barr virus and its association with disease - a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi T., Huang L., Luo L., Yu Q., Tian J. Diagnostic value of serological and molecular biological tests for infectious mononucleosis by EBV in different age stages and course of the disease. J Med Virol. 2021;93:3824–3834. doi: 10.1002/jmv.26558. [DOI] [PubMed] [Google Scholar]
- 9.Kawada J.I., Kamiya Y., Sawada A., Iwatsuki K., Izutsu K., Torii Y., et al. Viral DNA loads in various blood components of patients with Epstein–Barr virus-positive T-cell/natural killer cell lymphoproliferative diseases. J Infect Dis. 2019;220:1307–1311. doi: 10.1093/infdis/jiz315. [DOI] [PubMed] [Google Scholar]
- 10.Gao L.W., Xie Z.D., Liu Y.Y., Wang Y., Shen K.L. Epidemiologic and clinical characteristics of infectious mononucleosis associated with Epstein–Barr virus infection in children in Beijing. China. World J Pediatr. 2011;7:45–49. doi: 10.1007/s12519-011-0244-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q., Hu Z., Zhang Q.H. Analysis of Epstein Barr virus infection in 761 hospitalized children. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15:183–186. [PubMed] [Google Scholar]
- 12.Liu W.J., Du K., Liu X.Z., Wang G.W., Zhou L., Chen M. The correlation between IgM and virus DNA in children with respiratory infection of EBV. Int J Lab Med. 2012;33:283–284. [Google Scholar]
- 13.Huang Y., Wei C., Zheng K., Zhao D. The impact of serological features in Chinese children with primary or past Epstein–Barr virus infections. Virol J. 2013;10:55. doi: 10.1186/1743-422X-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smatti M.K., Al-Sadeq D.W., Ali N.H., Pintus G., Abou-Saleh H., Nasrallah G.K. Epstein–Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol. 2018;8:211. doi: 10.3389/fonc.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter J.R., Taylor G.S., Thomas O.G., Jackson C., Lewis J.E.A., Stagg H.R. Predictors of Epstein–Barr virus serostatus in young people in England. BMC Infect Dis. 2019;19:1007. doi: 10.1186/s12879-019-4578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutt-Fletcher L.M. Epstein–Barr virus entry. J Virol. 2007;81:7825–7832. doi: 10.1128/JVI.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teramura T., Tabata Y., Yagi T., Morimoto A., Hibi S., Imashuku S. Quantitative analysis of cell-free Epstein–Barr virus genome copy number in patients with EBV-associated hemophagocytic lymphohistiocytosis. Leuk Lymphoma. 2002;43:173–179. doi: 10.1080/10428190210176. [DOI] [PubMed] [Google Scholar]
- 18.Kanakry J.A., Hegde A.M., Durand C.M., Massie A.B., Greer A.E., Ambinder R.F., et al. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood. 2016;127:2007–2017. doi: 10.1182/blood-2015-09-672030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request (2231365607@qq.com; shiting0915@126.com).