Figure 2.
Function of neonatal human monocytes and antigen-presenting cells at different stages of intracellular signaling pathways.
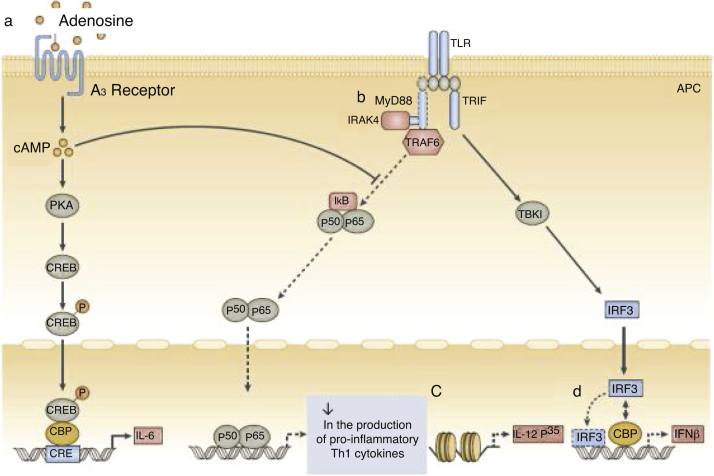
a, High concentrations of adenosine in neonatal blood plasma act through adenosine A3 receptors in neonatal mononuclear cells to induce high intracellular concentrations of cyclic AMP (cAMP). Dependent and independent protein kinase A (PKA) pathways can inhibit the tumor necrosis factor mediated by the Toll-like receptor 2 (TLR2), which preserves the production of interleukin-6 (IL-6). b, Neonatal monocytes have decreased expression of MyD88, a key adapter molecule for TLR-mediated signaling. c, Failure in the nucleosome remodeling of the Il12p35 gene promoter contributes to the decrease in TLR-mediated IL-12 p35 production by neonatal dendritic cells (DCs), an example of distinct regulation of neonatal cytokine production at the chromatin level. d, The lipopolysaccharide (LPS)-induced association of interferon (IFN) with regulatory factor 3 (IRF3) with cAMP-responsive element-binding protein (CREB) - binding protein (CBP) and DNA IRF3 binding are reduced in human neonates, resulting in impaired IFN receptor expression. Adapted from Levy6.