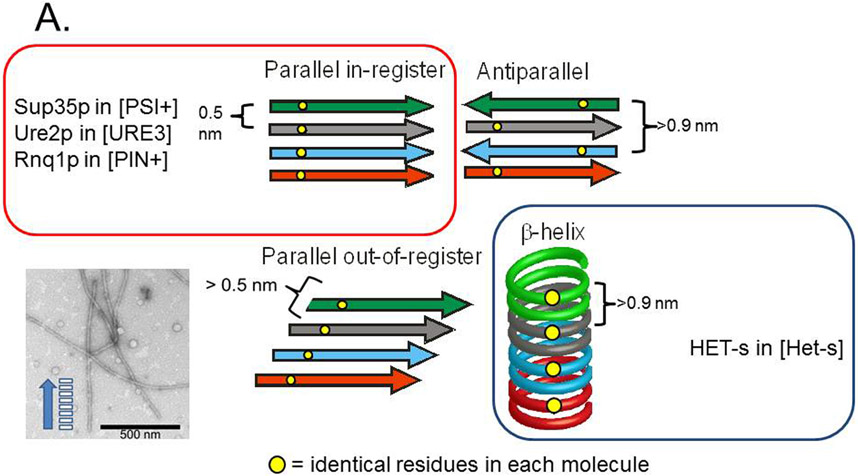
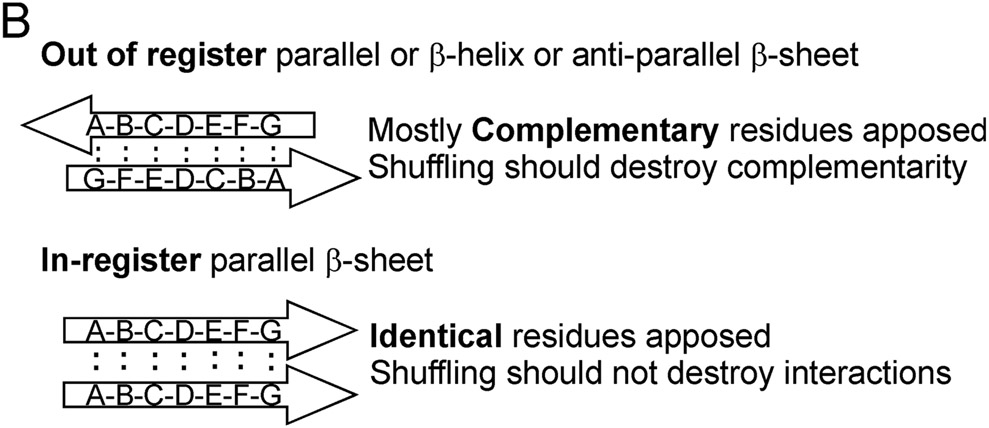
Fig. 4. Possible amyloid β-sheet structures.
A. Each color represents a separate molecule, and each small circle represents a particular atom, the same in each molecule. Labeling a single C atom with 13C and using solid-state NMR to measure the distance to the nearest neighbor 13C allows one to distinguish the in-register parallel β-sheet structure (~0.5 nm expected) from the other structures (> 0.5 nm expected). B. The strict sequence specificity for prion propagation implies an interaction between residues in the filament and those of a molecule newly joining the end. If the structure is antiparallel, β-helix, or out of register, that relationship must be one of complementarity. If the structure is in-register parallel, it is identity. Shuffling is expected to destroy the complementarity relationships (as it would with DNA strands), but not identity interactions, which could still all occur, just in a different order along the peptide chain (Ross et al. 2005). Modified from Ross et al., 2005.