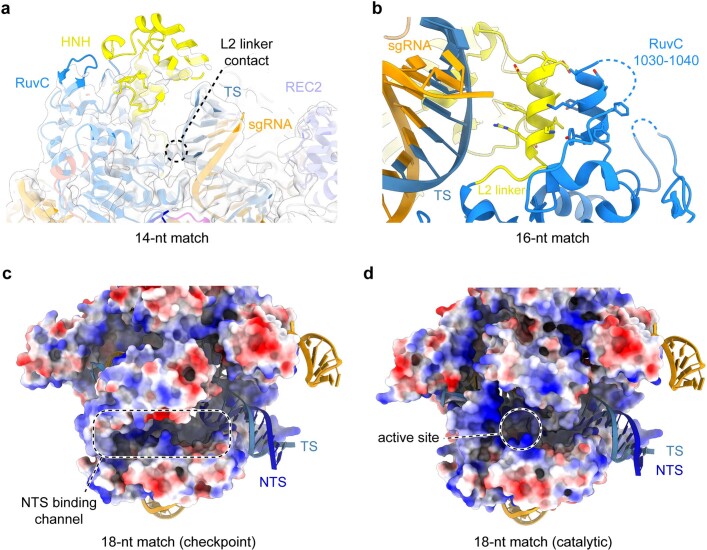
Extended Data Fig. 8. HNH undocking induced by R-loop extension.
a, Residual HNH domain density (white) observed in the 14-nt match complex, in which the elongated heteroduplex establishes a contact with the L2 linker. No NTS density is observed past the PAM region due to disorder. b, Zoom-in view of the interaction between the HNH domain L2 linker and the RuvC 1030–1040 helix induced by heteroduplex proximity of the 16-bp complex. c, HNH domain relocation towards the binding channel results in the formation of a positively charged NTS binding channel. No residual electron density (white) is observed for the NTS in the absence of the PAM-distal duplex. The protein is coloured according to electrostatic surface potential, with red being negative, blue positive. d, Surface electrostatics map of the 18-nt match catalytic state of SpCas9, showing the NTS binding cleft with cleaved NTS positioned within the active site.