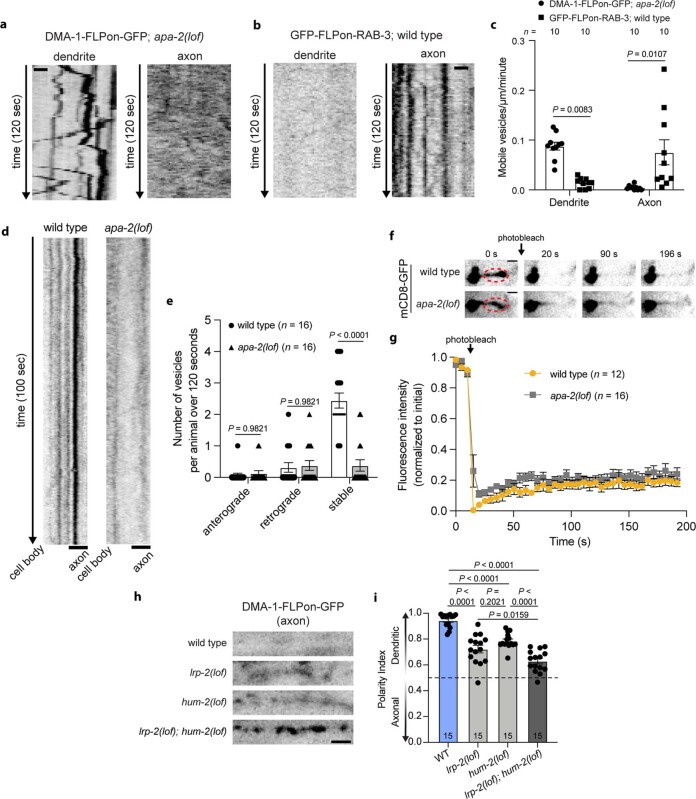
Extended Data Fig. 7. AIS endocytosis works in conjunction with known polarity mechanisms in the AIS to maintain neuronal polarity.
(a) Time-lapse imaging of cell-specific endogenous DMA-1-FLPon-GFP in apa-2 loss of function mutants shows robust vesicular trafficking in the dendrite but not axon. (b) Time-lapse imaging of cell-specific endogenous GFP-FLPon-RAB-3 shows robust vesicular localization and movement in the axon but not dendrite. (c) Quantification of vesicle dynamics described in a, b. (d) Kymograph analysis from time-lapse imaging of DMA-1-FLPon-GFP vesicles in the AIS of wild type and apa-2(lof) mutant animals. (e) Quantification of DMA-1-FLPon-GFP vesicular trafficking in the proximal AIS of wild type and apa-2(lof) endocytic mutant animals. (f) FRAP of mCD8-GFP in the AIS of wild type and apa-2(lof) endocytic mutants. P = 0.5842 using a two-way ANOVA to compare the two genotypes. (g) Fluorescence intensity of the red circled regions in f. Wild type data are from Extended Data Fig. 1i. (h) Cell-specific endogenous DMA-1-FLPon-GFP localization to the axon in the indicated animals. (i) DMA-1-FLPon-GFP polarity index of animals described in h. All data are shown as mean ± s.e.m. N-values are indicated on bar graphs and represent the number of individual animals scored for each condition. P values in c and e were calculated using a two-way ANOVA with Šidák multiple comparison test. P values in i were calculated using a one-way ANOVA with Šidák multiple comparison test. Scale bar, 2 µm (d, f, h), 1 µm (a, b).